| Molecular Interaction Atlas (MIA) |
|
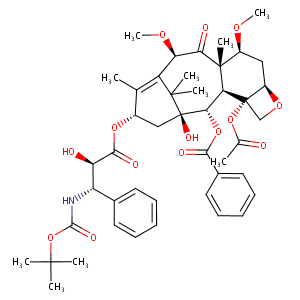
| Indication(s) of Cabazitaxel |
| Disease Entry |
ICD 11 |
Status |
REF |
| Breast cancer |
2C60-2C65
|
Approved |
[2] |
| Solid tumour/cancer |
2A00-2F9Z
|
Phase 2 |
[2] |
| Metastatic prostate carcinoma |
N.A.
|
Investigative |
[3] |
|
|
|
Cabazitaxel Interacts with 1 DTT Molecule(s)
| DTT Name |
DTT ID |
UniProt ID |
Mode of Action |
REF |
|
Tubulin (TUB)
|
TTML2WA
|
NOUNIPROTAC
|
Inhibitor
|
[4] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
|
|
Cabazitaxel Interacts with 3 DME Molecule(s)
| DME Name |
DME ID |
UniProt ID |
Mode of Action |
REF |
|
Cytochrome P450 3A4 (CYP3A4)
|
DE4LYSA
|
CP3A4_HUMAN
|
Metabolism
|
[5] |
|
Cytochrome P450 3A5 (CYP3A5)
|
DEIBDNY
|
CP3A5_HUMAN
|
Metabolism
|
[5] |
|
Cytochrome P450 2C8 (CYP2C8)
|
DES5XRU
|
CP2C8_HUMAN
|
Metabolism
|
[5] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
|
|
Cabazitaxel Interacts with 2 DOT Molecule(s)
| DOT Name |
DOT ID |
UniProt ID |
Mode of Action |
REF |
|
Forkhead box protein O3 (FOXO3)
|
OTHXQG4P
|
FOXO3_HUMAN
|
Affects Expression
|
[6] |
|
Proline-rich AKT1 substrate 1 (AKT1S1)
|
OT4JHN4Y
|
AKTS1_HUMAN
|
Decreases Expression
|
[6] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
|
|
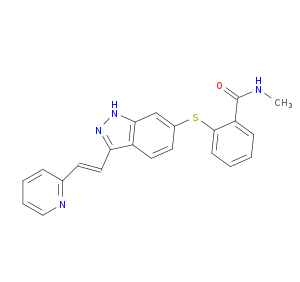
Indazole derivative 5 Interacts with 2 DTT Molecule(s)
| DTT Name |
DTT ID |
UniProt ID |
Mode of Action |
REF |
|
ABL T315I mutant (ABL T315I)
|
TTZJTWA
|
ABL1_HUMAN
|
Inhibitor
|
[7] |
|
Fusion protein Bcr-Abl T315I mutant (Bcr-Abl T315I)
|
TTIV39N
|
BCR_HUMAN-ABL1_HUMAN
|
Inhibitor
|
[7] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
|
|
|
|
|
|
|
|