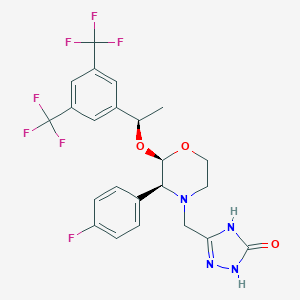
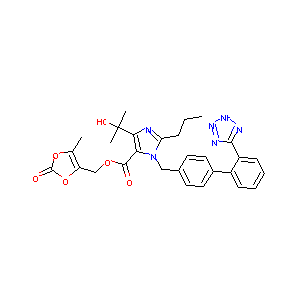
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3490).
|
| 4 |
Aprepitant FDA Label
|
| 5 |
ClinicalTrials.gov (NCT00571168) Efficacy and Safety of Aprepitant in Subjects With Multiple Myeloma During and After High-dose Chemotherapy. U.S. National Institutes of Health.
|
| 6 |
Effect of angiotensin receptor blockade on endothelial function: focus on olmesartan medoxomil. Vasc Health Risk Manag. 2009;5(1):301-14.
|
| 7 |
Potent, brain-penetrant, hydroisoindoline-based human neurokinin-1 receptor antagonists. J Med Chem. 2009 May 14;52(9):3039-46.
|
| 8 |
Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv Drug Deliv Rev. 2012 Jan;64(1):95-109.
|
| 9 |
Lack of effect of aprepitant on the pharmacokinetics of docetaxel in cancer patients. Cancer Chemother Pharmacol. 2005 Jun;55(6):609-16.
|
| 10 |
Cytochrome P450 3A4 is the major enzyme involved in the metabolism of the substance P receptor antagonist aprepitant. Drug Metab Dispos. 2004 Nov;32(11):1287-92.
|
| 11 |
Neurokinin-1 receptor (NK1R) inhibition sensitizes APL cells to anti-tumor effect of arsenic trioxide via restriction of NF-B axis: Shedding new light on resistance to Aprepitant. Int J Biochem Cell Biol. 2018 Oct;103:105-114. doi: 10.1016/j.biocel.2018.08.010. Epub 2018 Aug 23.
|
| 12 |
Mechanism of diastolic stiffening of the failing myocardium and its prevention by angiotensin receptor and calcium channel blockers. J Cardiovasc Pharmacol. 2009 Jul;54(1):47-56.
|
| 13 |
Multiple human isoforms of drug transporters contribute to the hepatic and renal transport of olmesartan, a selective antagonist of the angiotensin II AT1-receptor. Drug Metab Dispos. 2007 Dec;35(12):2166-76.
|
| 14 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01665)
|
| 15 |
KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01913)
|
| 16 |
OATP1B1, OATP1B3, and mrp2 are involved in hepatobiliary transport of olmesartan, a novel angiotensin II blocker. Drug Metab Dispos. 2006 May;34(5):862-9.
|
| 17 |
A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol Sci. 2013 Nov;136(1):216-41.
|
| 18 |
Immunopathogenesis of olmesartan-associated enteropathy. Aliment Pharmacol Ther. 2015 Dec;42(11-12):1303-14. doi: 10.1111/apt.13413. Epub 2015 Oct 1.
|
|
|
|
|
|
|