| 1 |
ClinicalTrials.gov (NCT01011036) Effects of GABA-a-Agonists on Pain Mechanisms: An Experimental Study in Healthy Volunteers
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
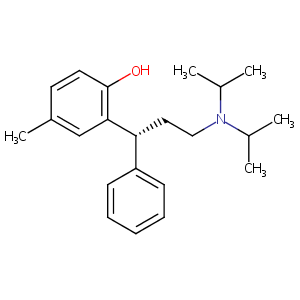
| 3 |
Tolterodine FDA Label
|
| 4 |
Clonazepam FDA Label
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6963).
|
| 6 |
Signal transduction underlying carbachol-induced contraction of human urinary bladder. J Pharmacol Exp Ther. 2004 Jun;309(3):1148-53.
|
| 7 |
Tolterodine, a new muscarinic receptor antagonist, is metabolized by cytochromes P450 2D6 and 3A in human liver microsomes. Drug Metab Dispos. 1998 Apr;26(4):289-93.
|
| 8 |
Effect of the CYP2D6*10 genotype on tolterodine pharmacokinetics. Drug Metab Dispos. 2010 Sep;38(9):1456-63.
|
| 9 |
Glutamate- and GABA-based CNS therapeutics. Curr Opin Pharmacol. 2006 Feb;6(1):7-17.
|
| 10 |
Identification of the human and animal hepatic cytochromes P450 involved in clonazepam metabolism. Fundam Clin Pharmacol. 1993;7(2):69-75.
|
| 11 |
Effect of common NAT2 variant alleles in the acetylation of the major clonazepam metabolite, 7-aminoclonazepam. Drug Metab Lett. 2007 Jan;1(1):3-5.
|
| 12 |
Characterization of Escherichia coli nitroreductase NfsB in the metabolism of nitrobenzodiazepines. Biochem Pharmacol. 2009 Jul 1;78(1):96-103.
|
|
|
|
|
|
|