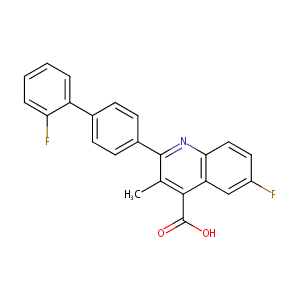
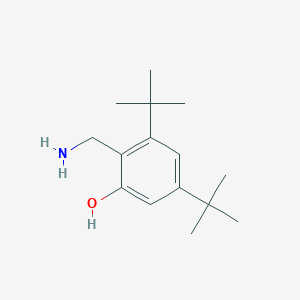
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
ClinicalTrials.gov (NCT04575038) CRISIS2: A Phase 2 Study of the Safety and Antiviral Activity of Brequinar in Non-hospitalized Pts With COVID-19 (CRISIS2). U.S. National Institutes of Health.
|
| 3 |
ClinicalTrials.gov (NCT03760666) A Study of Brequinar in Subjects With Relapsed/Refractory Acute Myeloid Leukemia. U.S. National Institutes of Health.
|
| 4 |
The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954.
|
| 5 |
DOI: 10.1203/00006450-198807000-00137
|
|
|
|
|
|
|