| 1 |
ClinicalTrials.gov (NCT01837394) Ultrasound-guided Blocks for Ambulatory Knee Arthroscopy
|
| 2 |
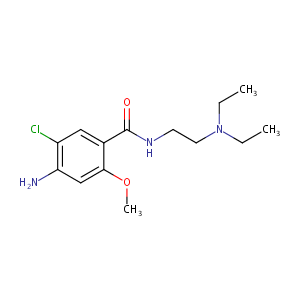
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 241).
|
| 3 |
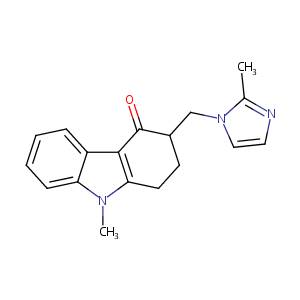
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2290).
|
| 4 |
Ondansetron FDA Label
|
| 5 |
Mechanisms for metoclopramide-mediated sensitization and haloperidol-induced catalepsy in rats. Eur J Pharmacol. 2008 Jun 10;587(1-3):181-6.
|
| 6 |
Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42.
|
| 7 |
Metoclopramide is metabolized by CYP2D6 and is a reversible inhibitor, but not inactivator, of CYP2D6. Xenobiotica. 2014 Apr;44(4):309-319.
|
| 8 |
Comparison of the effects of metoclopramide and domperidone on HERG channels. Pharmacology. 2005 Apr;74(1):31-6. doi: 10.1159/000083234. Epub 2005 Jan 7.
|
| 9 |
Exposure-based assessment of chemical teratogenicity using morphogenetic aggregates of human embryonic stem cells. Reprod Toxicol. 2020 Jan;91:74-91. doi: 10.1016/j.reprotox.2019.10.004. Epub 2019 Nov 8.
|
| 10 |
Differences in the opioid control of luteinizing hormone secretion between pathological and iatrogenic hyperprolactinemic states. J Clin Endocrinol Metab. 1987 Mar;64(3):508-12. doi: 10.1210/jcem-64-3-508.
|
| 11 |
Growth hormone and prolactin secretion after metoclopramide administration (DA2 receptor blockade) in fertile women. Horm Metab Res. 2001 Sep;33(9):536-9. doi: 10.1055/s-2001-17214.
|
| 12 |
Effects of metoclopramide on duodenal motility and flow events, glucose absorption, and incretin hormone release in response to intraduodenal glucose infusion. Am J Physiol Gastrointest Liver Physiol. 2010 Dec;299(6):G1326-33. doi: 10.1152/ajpgi.00476.2009. Epub 2010 Sep 9.
|
| 13 |
Effect of drugs interacting with the dopaminergic receptors on glucose levels and insulin release in healthy and type 2 diabetic subjects. Am J Ther. 2008 Jul-Aug;15(4):397-402. doi: 10.1097/MJT.0b013e318160c353.
|
| 14 |
Interactions of metoclopramide and ergotamine with human 5-HT(3A) receptors and human 5-HT reuptake carriers. Br J Pharmacol. 2005 Oct;146(4):543-52. doi: 10.1038/sj.bjp.0706351.
|
| 15 |
Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167.
|
| 16 |
Association of ABCB1, 5-HT3B receptor and CYP2D6 genetic polymorphisms with ondansetron and metoclopramide antiemetic response in Indonesian cancer patients treated with highly emetogenic chemotherapy. Jpn J Clin Oncol. 2011 Oct;41(10):1168-76. doi: 10.1093/jjco/hyr117. Epub 2011 Aug 11.
|
| 17 |
Clinical response and side effects of metoclopramide: associations with clinical, demographic, and pharmacogenetic parameters. J Clin Gastroenterol. 2012 Jul;46(6):494-503. doi: 10.1097/MCG.0b013e3182522624.
|
| 18 |
Treatment of pruritus in chronic liver disease with the 5-hydroxytryptamine receptor type 3 antagonist ondansetron: a randomized, placebo-controlled, double-blind cross-over trial. Eur J Gastroenterol Hepatol. 1998 Oct;10(10):865-70.
|
| 19 |
Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004 Jan;75(1):13-33.
|
| 20 |
The effect of rifampin on the pharmacokinetics of oral and intravenous ondansetron. Clin Pharmacol Ther. 1999 Apr;65(4):377-81.
|
| 21 |
Effects of serotonin-3 receptor antagonists on cytochrome P450 activities in human liver microsomes. Biol Pharm Bull. 2006 Sep;29(9):1931-5.
|
| 22 |
Cytochrome P450 2D6 metabolism and 5-hydroxytryptamine type 3 receptor antagonists for postoperative nausea and vomiting. Med Sci Monit. 2005 Oct;11(10):RA322-8.
|
| 23 |
Multiple forms of cytochrome P450 are involved in the metabolism of ondansetron in humans. Drug Metab Dispos. 1995 Nov;23(11):1225-30.
|
| 24 |
Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of dolasetron. Comparison with other indole-containing 5-HT3 antagonists. Drug Metab Dispos. 1996 May;24(5):602-9.
|
| 25 |
Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448.
|
| 26 |
Serotonin type-3 receptor antagonists selectively kill melanoma cells through classical apoptosis, microtubule depolymerisation, ERK activation, and NF-B downregulation. Cell Biol Toxicol. 2023 Jun;39(3):1119-1135. doi: 10.1007/s10565-021-09667-0. Epub 2021 Oct 15.
|
| 27 |
ClinicalTrials.gov (NCT02959840) Acupuncture Point P6 Stimulation for Reduction of Nausea and Vomiting During Cesarean
|
|
|
|
|
|
|