| 1 |
ClinicalTrials.gov (NCT02106507) ARN 509 Plus Everolimus in Men With Progressive Metastatic Castration-Resistant Prostate Cancer After Treatment With Abiraterone Acetate
|
| 2 |
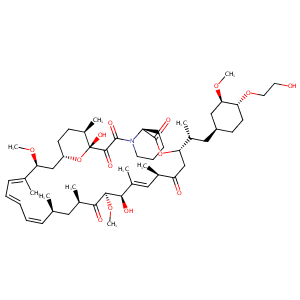
Everolimus FDA Label
|
| 3 |
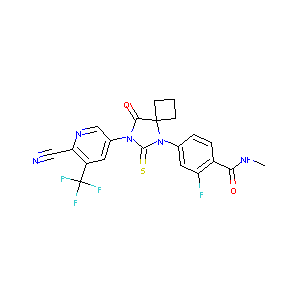
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5889).
|
| 5 |
Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 May;15(5):327-47.
|
| 6 |
2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89.
|
| 7 |
ClinicalTrials.gov (NCT01946204) A Study of ARN-509 in Men With Non-Metastatic Castration-Resistant Prostate Cancer. U.S. National Institutes of Health.
|
| 8 |
Mammalian target of rapamycin, its mode of action and clinical response in metastatic clear cell carcinoma. Gan To Kagaku Ryoho. 2009 Jul;36(7):1076-9.
|
| 9 |
Closer to the Site of Action: Everolimus Concentrations in Peripheral Blood Mononuclear Cells Correlate Well With Whole Blood Concentrations. Ther Drug Monit. 2015 Oct;37(5):675-80.
|
| 10 |
The evolving experience using everolimus in clinical transplantation. Transplant Proc. 2004 Mar;36(2 Suppl):495S-499S.
|
| 11 |
Apalutamide: first global approval. Drugs. 2018 Apr;78(6):699-705.
|
|
|
|
|
|
|