| 1 |
ClinicalTrials.gov (NCT02499562) A Phase II Clinical Trial of Hydronidone Capsules(F351) in Patients With Liver Fibrosis Induced by HBV Chronic Hepatitis
|
| 2 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 3 |
Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020436)
|
| 4 |
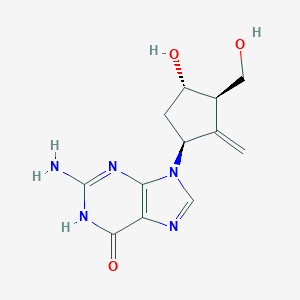
Effect of hepatitis B virus reverse transcriptase variations on entecavir treatment response. J Infect Dis. 2014 Sep 1;210(5):701-7.
|
| 5 |
Multiple SLC and ABC Transporters Contribute to the Placental Transfer of Entecavir. Drug Metab Dispos. 2017 Mar;45(3):269-278.
|
| 6 |
Human organic anion transporter 2 is an entecavir, but not tenofovir, transporter. Drug Metab Pharmacokinet. 2017 Feb;32(1):116-119.
|
| 7 |
The oligopeptide transporter 2-mediated reabsorption of entecavir in rat kidney. Eur J Pharm Sci. 2014 Feb 14;52:41-7.
|
| 8 |
Expansion of the clavulanic acid gene cluster: identification and in vivo functional analysis of three new genes required for biosynthesis of clavulanic acid by Streptomyces clavuligerus. J Bacteriol. 2000 Jul;182(14):4087-95.
|
|
|
|
|
|
|