| 1 |
ClinicalTrials.gov (NCT02787057) Vancomycin Plus Moxifloxacin Versus Vancomycin Plus Ceftazidime for the Treatment of Peritoneal Dialysis (PD)-Related Peritonitis
|
| 2 |
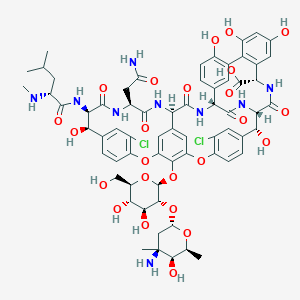
Vancomycin FDA Label
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954.
|
| 5 |
Novel agents in the management of Mycobacterium tuberculosis disease. Curr Med Chem. 2007;14(18):2000-8.
|
| 6 |
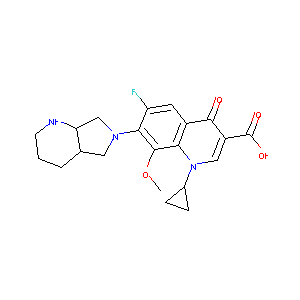
Moxifloxacin FDA Label
|
| 7 |
Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob Agents Chemother. 1993 Aug;37(8):1563-71.
|
| 8 |
Sequence of the vanC gene of Enterococcus gallinarum BM4174 encoding a D-alanine:D-alanine ligase-related protein necessary for vancomycin resistance. Gene. 1992 Mar 1;112(1):53-8.
|
| 9 |
vanG element insertions within a conserved chromosomal site conferring vancomycin resistance to Streptococcus agalactiae and Streptococcus anginosus. mBio. 2014 Jul 22;5(4):e01386-14.
|
| 10 |
Bacterial resistance to vancomycin: overproduction, purification, and characterization of VanC2 from Enterococcus casseliflavus as a D-Ala-D-Ser ligase. Proc Natl Acad Sci U S A. 1997 Sep 16;94(19):10040-4.
|
| 11 |
vanI: a novel D-Ala-D-Lac vancomycin resistance gene cluster found in Desulfitobacterium hafniense. Microb Biotechnol. 2014 Sep;7(5):456-66.
|
| 12 |
Emergence of high-level resistance to glycopeptides in Enterococcus gallinarum and Enterococcus casseliflavus. Antimicrob Agents Chemother. 1994 Jul;38(7):1675-7.
|
| 13 |
vanA in Enterococcus faecium, Enterococcus faecalis, and Enterococcus casseliflavus detected in French cattle. Foodborne Pathog Dis. 2009 Nov;6(9):1107-11.
|
| 14 |
Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. Chem Res Toxicol. 2015 May 18;28(5):927-34. doi: 10.1021/tx5005248. Epub 2015 Apr 3.
|
| 15 |
[Successful treatment of MRSA-associated glomerulonephritis with antibiotic therapy]. Nihon Jinzo Gakkai Shi. 2003;45(1):37-41.
|
| 16 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
|
|
|
|
|
|