| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Verteporfin FDA Label
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
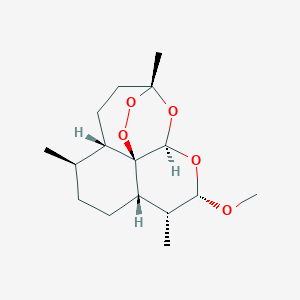
The fight against drug-resistant malaria: novel plasmodial targets and antimalarial drugs. Curr Med Chem. 2008;15(2):161-71.
|
| 5 |
The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin Cancer Res. 2007 Apr 15;13(8):2463-70.
|
| 6 |
Efficient purification and reconstitution of ATP binding cassette transporter B6 (ABCB6) for functional and structural studies. J Biol Chem. 2013 Aug 2;288(31):22658-69.
|
| 7 |
The effect of grapefruit juice on the time-dependent decline of artemether plasma levels in healthy subjects. Clin Pharmacol Ther. 1999 Oct;66(4):408-14.
|
| 8 |
The contribution of the enzymes CYP2D6 and CYP2C19 in the demethylation of artemether in healthy subjects. Eur J Drug Metab Pharmacokinet. 1998 Jul-Sep;23(3):429-36.
|
| 9 |
Effect of pharmacogenetics on plasma lumefantrine pharmacokinetics and malaria treatment outcome in pregnant women. Malar J. 2017 Jul 3;16(1):267.
|
| 10 |
Pharmacokinetic interaction between etravirine or darunavir/ritonavir and artemether/lumefantrine in healthy volunteers: a two-panel, two-way, two-period, randomized trial. HIV Med. 2013 Aug;14(7):421-9.
|
| 11 |
Insights into CYP2B6-mediated drug-drug interactions. Acta Pharm Sin B. 2016 Sep;6(5):413-425.
|
| 12 |
Fetal bovine serum and human constitutive androstane receptor: evidence for activation of the SV23 splice variant by artemisinin, artemether, and arteether in a serum-free cell culture system. Toxicol Appl Pharmacol. 2014 Jun 1;277(2):221-30. doi: 10.1016/j.taap.2014.03.023. Epub 2014 Apr 12.
|
|
|
|
|
|
|