| 1 |
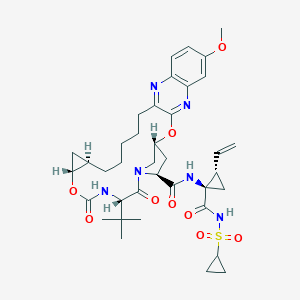
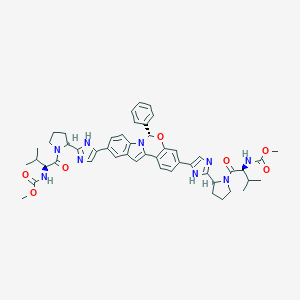
ClinicalTrials.gov (NCT02332707) Efficacy and Safety of Grazoprevir (MK-5172) and Uprifosbuvir (MK-3682) With Elbasvir (MK-8742) or Ruzasvir (MK-8408) for Chronic Hepatitis C Genotype (GT)1 and GT2 Infection (MK-3682-011)
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 3 |
Drug Repurposing of Approved Drugs Elbasvir, Ledipasvir, Paritaprevir, Velpatasvir, Antrafenine and Ergotamine for Combating COVID19. April 21, 2020.
|
| 4 |
FDA label of Elbasvir and grazoprevir. The 2020 official website of the U.S. Food and Drug Administration.
|
| 5 |
Clinical pharmacokinetics and drug-drug interactions of Elbasvir/Grazoprevir. Eur J Drug Metab Pharmacokinet. 2018 Oct;43(5):509-531.
|
| 6 |
2016 FDA drug approvals. Nat Rev Drug Discov. 2017 Feb 2;16(2):73-76.
|
| 7 |
ClinicalTrials.gov (NCT01717326) A Study of the Combination Regimen Grazoprevir (MK-5172) and Elbasvir (MK-8742) Ribavirin in Participants With Chronic Hepatitis C (MK-5172-035)
|
|
|
|
|
|
|