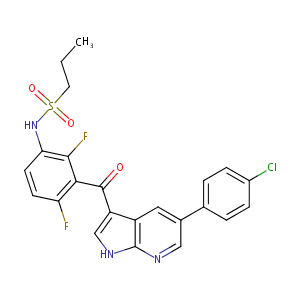
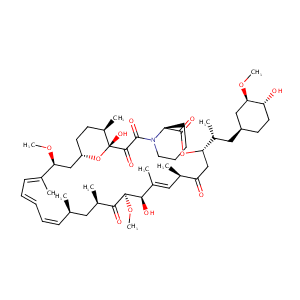
| 1 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5893).
|
| 3 |
Sirolimus FDA Label
|
| 4 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 201578.
|
| 5 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 6 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6031).
|
| 7 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 8 |
ClinicalTrials.gov (NCT04341675) Sirolimus Treatment in Hospitalized Patients With COVID-19 Pneumonia. U.S. National Institutes of Health.
|
| 9 |
PLX4032 Mediated Melanoma Associated Antigen Potentiation in Patient Derived Primary Melanoma Cells. J Cancer. 2015 Oct 29;6(12):1320-30. doi: 10.7150/jca.11126. eCollection 2015.
|
| 10 |
Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. EMBO J. 2016 Mar 1;35(5):462-78. doi: 10.15252/embj.201592081. Epub 2015 Dec 14.
|
| 11 |
Differential effects of the oncogenic BRAF inhibitor PLX4032 (vemurafenib) and its progenitor PLX4720 on ABCB1 function. J Pharm Pharm Sci. 2014;17(1):154-68.
|
| 12 |
Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin Cancer Res. 2013 Mar 15;19(6):1458-66.
|
| 13 |
Vemurafenib for the treatment of melanoma. Expert Opin Pharmacother. 2012 Dec;13(17):2533-43.
|
| 14 |
Perturbation biology nominates upstream-downstream drug combinations in RAF inhibitor resistant melanoma cells. Elife. 2015 Aug 18;4:e04640. doi: 10.7554/eLife.04640.
|
| 15 |
Role of the protein kinase BRAF in the pathogenesis of endometriosis. Expert Opin Ther Targets. 2016 Aug;20(8):1017-29. doi: 10.1080/14728222.2016.1180367. Epub 2016 May 4.
|
| 16 |
Overcoming melanoma resistance to vemurafenib by targeting CCL2-induced miR-34a, miR-100 and miR-125b. Oncotarget. 2016 Jan 26;7(4):4428-41. doi: 10.18632/oncotarget.6599.
|
| 17 |
Melanoma Expressed-CD70 Is Regulated by RhoA and MAPK Pathways without Affecting Vemurafenib Treatment Activity. PLoS One. 2016 Feb 1;11(2):e0148095. doi: 10.1371/journal.pone.0148095. eCollection 2016.
|
| 18 |
Sustained SREBP-1-dependent lipogenesis as a key mediator of resistance to BRAF-targeted therapy. Nat Commun. 2018 Jun 27;9(1):2500. doi: 10.1038/s41467-018-04664-0.
|
| 19 |
MAPK and SHH pathways modulate type 3 deiodinase expression in papillary thyroid carcinoma. Endocr Relat Cancer. 2016 Mar;23(3):135-46. doi: 10.1530/ERC-15-0162.
|
| 20 |
Paradoxical activation of MEK/ERK signaling induced by B-Raf inhibition enhances DR5 expression and DR5 activation-induced apoptosis in Ras-mutant cancer cells. Sci Rep. 2016 May 25;6:26803. doi: 10.1038/srep26803.
|
| 21 |
The BRAFT1799A mutation confers sensitivity of thyroid cancer cells to the BRAFV600E inhibitor PLX4032 (RG7204). Biochem Biophys Res Commun. 2011 Jan 28;404(4):958-62. doi: 10.1016/j.bbrc.2010.12.088. Epub 2010 Dec 23.
|
| 22 |
HSP70 Inhibition Limits FAK-Dependent Invasion and Enhances the Response to Melanoma Treatment with BRAF Inhibitors. Cancer Res. 2016 May 1;76(9):2720-30. doi: 10.1158/0008-5472.CAN-15-2137. Epub 2016 Mar 16.
|
| 23 |
Knockouts model the 100 best-selling drugs--will they model the next 100 Nat Rev Drug Discov. 2003 Jan;2(1):38-51.
|
| 24 |
The genomic landscape of nasopharyngeal carcinoma.Nat Genet. 2014 Aug;46(8):866-71.
|
| 25 |
Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020 Mar 16;6:14.
|
| 26 |
Pharmacogenetics of tacrolimus and sirolimus in renal transplant patients: from retrospective analyses to prospective studies. Transplant Proc. 2007 Sep;39(7):2142-4.
|
| 27 |
Pharmacokinetic and pharmacodynamic interactions between the immunosuppressant sirolimus and the lipid-lowering drug ezetimibe in healthy volunteers. Clin Pharmacol Ther. 2010 Jun;87(6):663-7.
|
| 28 |
Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448.
|
| 29 |
Drug Interactions Flockhart Table
|
| 30 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 31 |
Biologically active neutrophil chemokine pattern in tonsillitis.Clin Exp Immunol. 2004 Mar;135(3):511-8. doi: 10.1111/j.1365-2249.2003.02390.x.
|
|
|
|
|
|
|