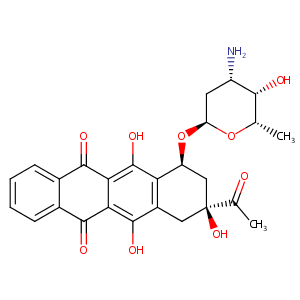
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Idarubicin FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7083).
|
| 4 |
Isoniazid FDA Label
|
| 5 |
Novel agents in the management of Mycobacterium tuberculosis disease. Curr Med Chem. 2007;14(18):2000-8.
|
| 6 |
Quantitative high-throughput profiling of environmental chemicals and drugs that modulate farnesoid X receptor. Sci Rep. 2014 Sep 26;4:6437. doi: 10.1038/srep06437.
|
| 7 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 8 |
Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007.
|
| 9 |
Amonafide L-malate is not a substrate for multidrug resistance proteins in secondary acute myeloid leukemia. Leukemia. 2008 Nov;22(11):2110-5.
|
| 10 |
In vitro evaluation of cytochrome P450-mediated drug interactions between cytarabine, idarubicin, itraconazole and caspofungin. Hematology. 2004 Jun;9(3):217-21.
|
| 11 |
A Quantitative Approach to Screen for Nephrotoxic Compounds In Vitro. J Am Soc Nephrol. 2016 Apr;27(4):1015-28. doi: 10.1681/ASN.2015010060. Epub 2015 Aug 10.
|
| 12 |
The use of biochemical markers in cardiotoxicity monitoring in patients treated for leukemia. Neoplasma. 2005;52(5):430-4.
|
| 13 |
The induction of apoptosis by daunorubicin and idarubicin in human trisomic and diabetic fibroblasts. Cell Mol Biol Lett. 2008;13(2):182-94. doi: 10.2478/s11658-007-0045-7. Epub 2008 Apr 10.
|
| 14 |
Refining the human iPSC-cardiomyocyte arrhythmic risk assessment model. Toxicol Sci. 2013 Dec;136(2):581-94. doi: 10.1093/toxsci/kft205. Epub 2013 Sep 19.
|
| 15 |
Characterization of drug-specific signaling between primary human hepatocytes and immune cells. Toxicol Sci. 2017 Jul 1;158(1):76-89.
|
| 16 |
Diversity in enoyl-acyl carrier protein reductases. Cell Mol Life Sci. 2009 May;66(9):1507-17.
|
| 17 |
Inhibition of CYP2E1 catalytic activity in vitro by S-adenosyl-L-methionine. Biochem Pharmacol. 2005 Apr 1;69(7):1081-93.
|
| 18 |
Crystal structure of the catalase-peroxidase KatG W78F mutant from Synechococcus elongatus PCC7942 in complex with the antitubercular pro-drug isoniazid. FEBS Lett. 2015 Jan 2;589(1):131-7.
|
| 19 |
The actinobacterium Tsukamurella paurometabola has a functionally divergent arylamine N-acetyltransferase (NAT) homolog. World J Microbiol Biotechnol. 2019 Oct 31;35(11):174.
|
| 20 |
Quercetin protected against isoniazide-induced HepG2 cell apoptosis by activating the SIRT1/ERK pathway. J Biochem Mol Toxicol. 2019 Sep;33(9):e22369. doi: 10.1002/jbt.22369. Epub 2019 Jul 23.
|
| 21 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 22 |
Mechanism-based inactivation of human cytochrome P4502C8 by drugs in vitro. J Pharmacol Exp Ther. 2004 Dec;311(3):996-1007.
|
| 23 |
Determination of phospholipidosis potential based on gene expression analysis in HepG2 cells. Toxicol Sci. 2007 Mar;96(1):101-14.
|
| 24 |
Comparison of base-line and chemical-induced transcriptomic responses in HepaRG and RPTEC/TERT1 cells using TempO-Seq. Arch Toxicol. 2018 Aug;92(8):2517-2531.
|
| 25 |
Identification of differentially expressed genes in hepatic HepG2 cells treated with acetaminophen using suppression subtractive hybridization. Biol Pharm Bull. 2005 Jul;28(7):1148-53. doi: 10.1248/bpb.28.1148.
|
| 26 |
Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020 Dec;94(12):4037-4041. doi: 10.1007/s00204-020-02869-1. Epub 2020 Aug 17.
|
| 27 |
An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20.
|
| 28 |
Auto-oxidation of Isoniazid Leads to Isonicotinic-Lysine Adducts on Human Serum Albumin. Chem Res Toxicol. 2015 Jan 20;28(1):51-8. doi: 10.1021/tx500285k. Epub 2014 Dec 9.
|
| 29 |
Isoniazid-induced apoptosis in HepG2 cells: generation of oxidative stress and Bcl-2 down-regulation. Toxicol Mech Methods. 2010 Jun;20(5):242-51. doi: 10.3109/15376511003793325.
|
| 30 |
The Isoniazid Metabolites Hydrazine and Pyridoxal Isonicotinoyl Hydrazone Modulate Heme Biosynthesis. Toxicol Sci. 2019 Mar 1;168(1):209-224. doi: 10.1093/toxsci/kfy294.
|
| 31 |
Isoniazid suppresses antioxidant response element activities and impairs adipogenesis in mouse and human preadipocytes. Toxicol Appl Pharmacol. 2013 Dec 15;273(3):435-41. doi: 10.1016/j.taap.2013.10.005. Epub 2013 Oct 12.
|
| 32 |
AMPK activator acadesine fails to alleviate isoniazid-caused mitochondrial instability in HepG2 cells. J Appl Toxicol. 2017 Oct;37(10):1219-1224. doi: 10.1002/jat.3483. Epub 2017 May 29.
|
| 33 |
Enhanced activation of human NK cells by drug-exposed hepatocytes. Arch Toxicol. 2020 Feb;94(2):439-448. doi: 10.1007/s00204-020-02668-8. Epub 2020 Feb 14.
|
| 34 |
Effects of N-acetyltransferase 2 (NAT2), CYP2E1 and Glutathione-S-transferase (GST) genotypes on the serum concentrations of isoniazid and metabolites in tuberculosis patients. J Toxicol Sci. 2008 May;33(2):187-95. doi: 10.2131/jts.33.187.
|
| 35 |
Eosinophil peroxidase oxidizes isoniazid to form the active metabolite against M. tuberculosis, isoniazid-NAD(). Chem Biol Interact. 2019 May 25;305:48-53. doi: 10.1016/j.cbi.2019.03.019. Epub 2019 Mar 25.
|
| 36 |
Metabolism of isoniazid by neutrophil myeloperoxidase leads to isoniazid-NAD(+) adduct formation: A comparison of the reactivity of isoniazid with its known human metabolites. Biochem Pharmacol. 2016 Apr 15;106:46-55. doi: 10.1016/j.bcp.2016.02.003. Epub 2016 Feb 9.
|
| 37 |
Development of a highly sensitive cytotoxicity assay system for CYP3A4-mediated metabolic activation. Drug Metab Dispos. 2011 Aug;39(8):1388-95. doi: 10.1124/dmd.110.037077. Epub 2011 May 3.
|
| 38 |
Customised in vitro model to detect human metabolism-dependent idiosyncratic drug-induced liver injury. Arch Toxicol. 2018 Jan;92(1):383-399. doi: 10.1007/s00204-017-2036-4. Epub 2017 Jul 31.
|
| 39 |
Biologically active neutrophil chemokine pattern in tonsillitis.Clin Exp Immunol. 2004 Mar;135(3):511-8. doi: 10.1111/j.1365-2249.2003.02390.x.
|
| 40 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
|
|
|
|
|
|