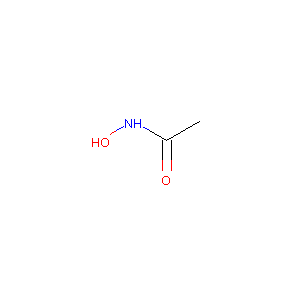
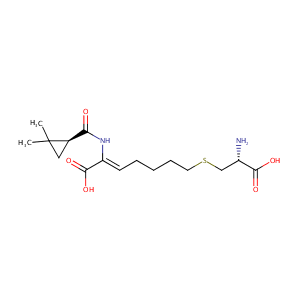
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 018749.
|
| 3 |
Cilastatin FDA Label
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5166).
|
| 5 |
Enzymatic, immunological and phylogenetic characterization of Brucella suis urease. BMC Microbiol. 2008 Jul 19;8:121.
|
| 6 |
New bispyridinium oximes: in vitro and in vivo evaluation of their biological efficiency in soman and tabun poisoning. Chem Biol Interact. 2008 Sep 25;175(1-3):413-6.
|
| 7 |
Pharmacokinetic study of pleural fluid penetration of carbapenem antibiotic agents in chemical pleurisy. Respir Med. 2006 Feb;100(2):324-31.
|
| 8 |
Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet. 2019 Feb;51(2):230-236.
|
|
|
|
|
|
|