| 1 |
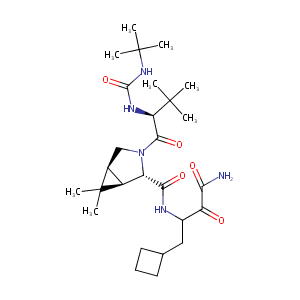
ClinicalTrials.gov (NCT01288417) Pharmacokinetic Study of the HCV Protease Inhibitor Boceprevir and the HIV Integrase Inhibitor Raltegravir
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7876).
|
| 3 |
Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. 2020. April 20. doi: https://doi.org/10.1101/2020.04.20.051581
|
| 4 |
2007 FDA drug approvals: a year of flux. Nat Rev Drug Discov. 2008 Feb;7(2):107-9.
|
| 5 |
2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4.
|
| 6 |
Drug interactions and protease inhibitors used in the treatment of hepatitis C: how to manage? Eur J Clin Pharmacol. 2014 Jul;70(7):775-89.
|
| 7 |
Pharmacokinetic evaluation of the interaction between hepatitis C virus protease inhibitor boceprevir and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors atorvastatin and pravastatin. Antimicrob Agents Chemother. 2013 Jun;57(6):2582-8.
|
| 8 |
Direct-acting antiviral agents for hepatitis C virus infection. Annu Rev Pharmacol Toxicol. 2013;53:427-49.
|
| 9 |
Population pharmacokinetic analysis and pharmacogenetics of raltegravir in HIV-positive and healthy individuals. Antimicrob Agents Chemother. 2012 Jun;56(6):2959-66. doi: 10.1128/AAC.05424-11. Epub 2012 Feb 27.
|
| 10 |
Successful tacrolimus treatment following renal transplant in a HIV-infected patient with raltegravir previously treated with a protease inhibitor based regimen. Drug Metabol Drug Interact. 2011;26(3):139-41.
|
| 11 |
Exposure-related effects of atazanavir on the pharmacokinetics of raltegravir in HIV-1-infected patients. Ther Drug Monit. 2010 Dec;32(6):782-6.
|
|
|
|
|
|
|