| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
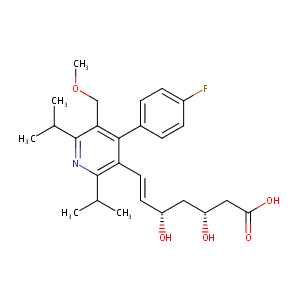
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2950).
|
| 3 |
Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs. 2009 Mar;14(1):99-127.
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6687).
|
| 5 |
Identification of the hepatic efflux transporters of organic anions using double-transfected Madin-Darby canine kidney II cells expressing human organic anion-transporting polypeptide 1B1 (OATP1B1)/multidrug resistance-associated protein 2, OATP1B1/multidrug resistance 1, and OATP1B1/breast cancer resistance protein. J Pharmacol Exp Ther. 2005 Sep;314(3):1059-67.
|
| 6 |
Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64.
|
| 7 |
FDA Drug Development and Drug Interactions
|
| 8 |
Differential effect of genetic variants of Na(+)-taurocholate co-transporting polypeptide (NTCP) and organic anion-transporting polypeptide 1B1 (OATP1B1) on the uptake of HMG-CoA reductase inhibitors. Xenobiotica. 2011 Jan;41(1):24-34.
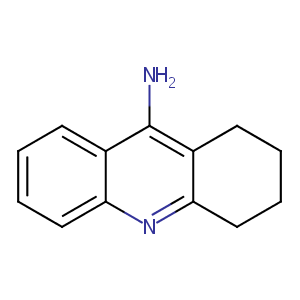
|
| 9 |
pH-sensitive interaction of HMG-CoA reductase inhibitors (statins) with organic anion transporting polypeptide 2B1. Mol Pharm. 2011 Aug 1;8(4):1303-13.
|
| 10 |
Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675.
|
| 11 |
Cerivastatin, genetic variants, and the risk of rhabdomyolysis. Pharmacogenet Genomics. 2011 May;21(5):280-8.
|
| 12 |
Drug Interactions Flockhart Table
|
| 13 |
Role of cytochrome P450 2C8 in drug metabolism and interactions. Pharmacol Rev. 2016 Jan;68(1):168-241.
|
| 14 |
Cerivastatin, genetic variants, and the risk of rhabdomyolysis. Pharmacogenet Genomics. 2011 May;21(5):280-8.
|
| 15 |
Evidence that the clinical effects of cholinesterase inhibitors are related to potency and targeting of action. Int J Clin Pract Suppl. 2002 Jun;(127):6-19.
|
| 16 |
Tacrine sinusoidal uptake and biliary excretion in sandwich-cultured primary rat hepatocytes. J Pharm Pharm Sci. 2014;17(3):427-38.
|
| 17 |
Synthetic and natural compounds that interact with human cytochrome P450 1A2 and implications in drug development. Curr Med Chem. 2009;16(31):4066-218.
|
| 18 |
Combined glutathione-S-transferase M1 and T1 genetic polymorphism and tacrine hepatotoxicity. Clin Pharmacol Ther. 2000 Apr;67(4):432-7.
|
| 19 |
Comparative effects of cationic triarylmethane, phenoxazine and phenothiazine dyes on horse serum butyrylcholinesterase. Arch Biochem Biophys. 2008 Oct 15;478(2):201-5.
|
| 20 |
Inhibition of human carboxylesterases hCE1 and hiCE by cholinesterase inhibitors. Chem Biol Interact. 2013 Mar 25;203(1):226-30.
|
| 21 |
Reduction and scavenging of chemically reactive drug metabolites by NAD(P)H:quinone oxidoreductase 1 and NRH:quinone oxidoreductase 2 and variability in hepatic concentrations. Chem Res Toxicol. 2018 Feb 19;31(2):116-126.
|
| 22 |
Evaluating the Role of Multidrug Resistance Protein 3 (MDR3) Inhibition in Predicting Drug-Induced Liver Injury Using 125 Pharmaceuticals. Chem Res Toxicol. 2017 May 15;30(5):1219-1229. doi: 10.1021/acs.chemrestox.7b00048. Epub 2017 May 4.
|
| 23 |
Correlation of brain levels of 9-amino-1,2,3,4-tetrahydroacridine (THA) with neurochemical and behavioral changes. Eur J Pharmacol. 1989 Nov 28;173(1):53-64. doi: 10.1016/0014-2999(89)90008-3.
|
| 24 |
Crystal structure of human carboxylesterase 1 complexed with the Alzheimer's drug tacrine: from binding promiscuity to selective inhibition. Chem Biol. 2003 Apr;10(4):341-9. doi: 10.1016/s1074-5521(03)00071-1.
|
| 25 |
High-content imaging-based BAC-GFP toxicity pathway reporters to assess chemical adversity liabilities. Arch Toxicol. 2017 Mar;91(3):1367-1383. doi: 10.1007/s00204-016-1781-0. Epub 2016 Jun 29.
|
| 26 |
Refining the human iPSC-cardiomyocyte arrhythmic risk assessment model. Toxicol Sci. 2013 Dec;136(2):581-94. doi: 10.1093/toxsci/kft205. Epub 2013 Sep 19.
|
| 27 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 28 |
Development of a highly sensitive cytotoxicity assay system for CYP3A4-mediated metabolic activation. Drug Metab Dispos. 2011 Aug;39(8):1388-95. doi: 10.1124/dmd.110.037077. Epub 2011 May 3.
|
|
|
|
|
|
|