| 1 |
ClinicalTrials.gov (NCT01789879) A Pharmacokinetic Evaluation of Levonorgestrel Implant and Antiretroviral Therapy
|
| 2 |
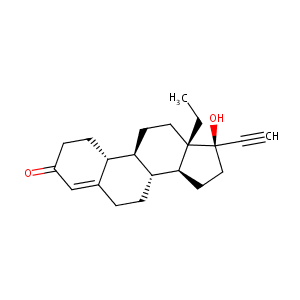
Levonorgestrel FDA Label
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2881).
|
| 4 |
Approved antiretroviral drugs. Antiretroviral Drugs. Company report of AVERT. 2009.
|
| 5 |
Effects of 17beta-estradiol, progesterone, synthetic progestins, tibolone, and raloxifene on vascular endothelial growth factor and Thrombospondin-1 messenger RNA in breast cancer cells. Int J Gynecol Cancer. 2006;16 Suppl 2:560-3. doi: 10.1111/j.1525-1438.2006.00696.x.
|
| 6 |
Met909 plays a key role in the activation of the progesterone receptor and also in the high potency of 13-ethyl progestins. Mol Pharmacol. 2009 Jun;75(6):1317-24.
|
| 7 |
P-glycoprotein (P-gp/MDR1)-mediated efflux of sex-steroid hormones and modulation of P-gp expression in vitro. Pharm Res. 2004 Jul;21(7):1284-93.
|
| 8 |
Progestins as inhibitors of the human 20-ketosteroid reductases, AKR1C1 and AKR1C3. Chem Biol Interact. 2011 May 30;191(1-3):227-33.
|
| 9 |
[Effect of nylestriol and levonorgestrel on the expression of Opg/OPGL in human osteosarcoma MG-63 cell lines]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2004 Dec;29(6):667-70.
|
| 10 |
Study of low-density lipoprotein receptor regulation by oral (steroid) contraceptives: desogestrel, levonorgestrel and ethinyl estradiol in JEG-3 cell line and placental tissue. Contraception. 2007 Oct;76(4):297-305. doi: 10.1016/j.contraception.2007.06.011. Epub 2007 Sep 14.
|
| 11 |
Relative progestational and androgenic activity of four progestins used for male hormonal contraception assessed in vitro in relation to their ability to suppress LH secretion in the castrate male rat. Mol Cell Endocrinol. 2010 Oct 26;328(1-2):16-21. doi: 10.1016/j.mce.2010.06.010. Epub 2010 Jun 25.
|
| 12 |
Serum distribution of the major metabolites of norgestimate in relation to its pharmacological properties. Contraception. 2003 Feb;67(2):93-9. doi: 10.1016/s0010-7824(02)00473-0.
|
| 13 |
Effects of natural products and nutraceuticals on steroid hormone-regulated gene expression. Clin Chim Acta. 2001 Oct;312(1-2):213-9. doi: 10.1016/s0009-8981(01)00626-x.
|
| 14 |
Effect of menstrual cycle and hormonal treatment on ki-67 and bcl-2 expression and adenomyosis. Gynecol Endocrinol. 2005 Mar;20(3):127-31. doi: 10.1080/09513590400021086.
|
| 15 |
Nevirapine restores androgen signaling in hormone-refractory human prostate carcinoma cells both in vitro and in vivo. Prostate. 2009 May 15;69(7):744-54. doi: 10.1002/pros.20923.
|
| 16 |
Reverse transcriptase inhibitors alter uncoupling protein-1 and mitochondrial biogenesis in brown adipocytes. Antivir Ther. 2005;10(4):515-26.
|
| 17 |
HIV-1 reverse transcriptase complex with DNA and nevirapine reveals non-nucleoside inhibition mechanism.Nat Struct Mol Biol.2012 Jan 22;19(2):253-9.
|
| 18 |
Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007.
|
| 19 |
Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab Dispos. 1999 Dec;27(12):1488-95.
|
| 20 |
Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675.
|
| 21 |
Helices F-G are important for the substrate specificities of CYP3A7. Drug Metab Dispos. 2007 Mar;35(3):484-92.
|
| 22 |
Inhibition of human cytochrome P450 isoforms by nonnucleoside reverse transcriptase inhibitors. J Clin Pharmacol. 2001 Jan;41(1):85-91.
|
| 23 |
RAT CYP3A and CYP2B1/2 were not associated with nevirapine-induced hepatotoxicity. Methods Find Exp Clin Pharmacol. 2006 Sep;28(7):423-31.
|
| 24 |
Bioactivation of nevirapine to a reactive quinone methide: implications for liver injury. Chem Res Toxicol. 2012 Aug 20;25(8):1708-19. doi: 10.1021/tx300172s. Epub 2012 Jul 26.
|
| 25 |
Transcriptional profiling suggests that Nevirapine and Ritonavir cause drug induced liver injury through distinct mechanisms in primary human hepatocytes. Chem Biol Interact. 2016 Aug 5;255:31-44.
|
| 26 |
An in vitro coculture system of human peripheral blood mononuclear cells with hepatocellular carcinoma-derived cells for predicting drug-induced liver injury. Arch Toxicol. 2021 Jan;95(1):149-168. doi: 10.1007/s00204-020-02882-4. Epub 2020 Aug 20.
|
| 27 |
Reverse transcriptase inhibitors down-regulate cell proliferation in vitro and in vivo and restore thyrotropin signaling and iodine uptake in human thyroid anaplastic carcinoma. J Clin Endocrinol Metab. 2005 Oct;90(10):5663-71. doi: 10.1210/jc.2005-0367. Epub 2005 Jul 19.
|
| 28 |
Identification of danger signals in nevirapine-induced skin rash. Chem Res Toxicol. 2013 Sep 16;26(9):1378-83. doi: 10.1021/tx400232s. Epub 2013 Aug 30.
|
| 29 |
Nevirapine increases high-density lipoprotein cholesterol concentration by stimulation of apolipoprotein A-I production. Arterioscler Thromb Vasc Biol. 2009 Sep;29(9):1336-41. doi: 10.1161/ATVBAHA.109.192088. Epub 2009 Aug 10.
|
| 30 |
Binding of anti-HIV drugs to human serum albumin. IUBMB Life. 2004 Oct;56(10):609-14. doi: 10.1080/15216540400016286.
|
| 31 |
Effects of nevirapine and efavirenz on human adipocyte differentiation, gene expression, and release of adipokines and cytokines. Antiviral Res. 2011 Aug;91(2):112-9. doi: 10.1016/j.antiviral.2011.04.018. Epub 2011 May 17.
|
| 32 |
Different Reactive Metabolites of Nevirapine Require Distinct Glutathione S-Transferase Isoforms for Bioinactivation. Chem Res Toxicol. 2016 Dec 19;29(12):2136-2144. doi: 10.1021/acs.chemrestox.6b00250. Epub 2016 Nov 28.
|
|
|
|
|
|
|