Details of the Drug Combinations
General Information of This Drug (ID: DM1UJO0)
| Drug Name | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Livalo; Pitavastatin calcium; 147526-32-7; Pitavastatin hemicalcium; NK-104; Nisvastatin; UNII-IYD54XEG3W; Flovas; IYD54XEG3W; 2C25H23FNO4Ca; CHEBI:71258; NK 104 (acid); Calcium (3R,5S,E)-7-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-3,5-dihydroxyhept-6-enoate; AK-50694; Itavastatin calcium; Bis((3R,5S,6E)-7-(2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl)-3,5-dihydroxy-6-heptenoate), monocalcium salt; Pitavastatin calcium (JAN); Alipza; Livazo; P-872441; P 872441; 6-Heptenoic acid,
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
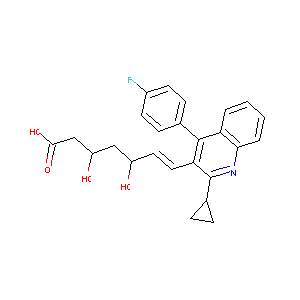 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||
