Details of the Drug Combinations
General Information of This Drug (ID: DM8JXPZ)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Abbenclamide; Adiab; Azuglucon; Bastiverit; Benclamin; Betanase; Calabren; Cytagon; Daonil; Debtan; Diabeta; Diabiphage; Dibelet; Duraglucon; Euclamin; Euglucan; Euglucon; Euglykon; Gewaglucon; Gilemal; Glamide; Glibadone; Gliban; Gliben; Glibenbeta; Glibenclamida; Glibenclamidum; Glibenil; Glibens; Glibesyn; Glibet; Glibetic; Glibil; Gliboral; Glicem; Glidiabet; Glimel; Glimide; Glimidstata; Glisulin; Glitisol; Glubate; Gluben; Glucobene; Glucohexal; Glucolon; Glucomid; Glucoremed; Glucoven; Glyben; Glybenclamide; Glybenzcyclamide; Glyburide; Glycolande; Glycomin; Glynase; Hexaglucon; Humedia; Lederglib; Libanil; Lisaglucon; Maninil; Melix; Micronase; Miglucan; Nadib; Neogluconin; Normoglucon; Orabetic; Pira; Praeciglucon; PresTab; Prodiabet; Renabetic; Sugril; Suraben; Tiabet; Yuglucon; Euglucon N; Glibenclamid AL; Glibenclamid Basics; Glibenclamid Fabra; Glibenclamid Genericon; Glibenclamid Heumann; Glibenclamid Riker M; Glyburide [USAN]; Micronized glyburide; Betanese 5; Euglucon 5; G 0639; GBN 5; HB 419; HB 420; HB419; HB420; Norglicem 5; U 26452; UR 606; Apo-Glibenclamide; Daonil (TN); Dia-basan; Diabeta (TN); Euglucon (TN); Gen-Glybe; Gliben-Puren N; Glibenclamid Riker M.; Glibenclamid-Cophar; Glibenclamid-Ratiopharm; Glibenclamida [INN-Spanish]; Glibenclamidum [INN-Latin]; Gluco-Tablimen; Glyburide (USP); Glyburide (micronized); Glynase (TN); HB-419; HB-420; Hemi-Daonil; Med-Glionil; Micronase (TN); Novo-Glyburide; Semi-Euglucon; Semi-daonil; U-26452; Glibenclamide (JP15/INN); Semi-Daonil (TN); Semi-Gliben-Puren N; N-p-[2-(5-Chloro-2-methoxybenzamido)ethyl]benzenesulfonyl-N'-cyclohexylurea; N-p-[2-(5-Chloro-2-methoxybenzamido)-ethyl]benzene-sulfonyl-N-cyclohexylurea; N-(4-(2-(5-Chloro-2-methoxybenzamido)ethyl)phenylsulfonyl)-N'-cyclohexylurea; 1-((p-(2-(5-Chloro-o-anisamido)ethyl)phenyl)sulfonyl)-3-cyclohexylurea; 1-(p-(2-(5-Chloro-2-methoxybenzamido)ethyl)benzenesulfonyl)-3-cyclohexylurea; 5-Chloro-N-[4-(cyclohexylureidosulfonyl)phenethyl]-2-methoxybenzamide; 5-chloro-N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-2-methoxybenzamide
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Hypoglycemic Agents
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
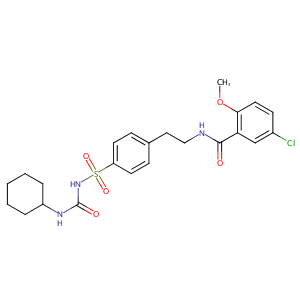 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
