Details of the Drug Combinations
General Information of This Drug (ID: DM8SMD1)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Avage; Suretin; Tazaroteno; Tazarotenum; Tazorac; Tazoral; Zorac; AGN 190168; AGN-190168; Avage (TN); Tazarotene [USAN:INN]; Tazorac (TN); Zorac (TN); Tazarotene (JAN/USAN/INN); Tazorac, Avage, Zora, Tazarotene; Ethyl 6-((4,4-dimethylthiochroman-6-yl)ethynyl)nicotinate; Ethyl 6-(2-(4,4-dimethylthiochroman-6-yl)ethynyl)nicotinate; Ethyl 6-[(4,4-dimethyl-3,4-dihydro-2H-thiochromen-6-yl)ethynyl]nicotinate; Ethyl 6-[2-(4,4-dimethyl-2,3-dihydrothiochromen-6-yl)ethynyl]pyridine-3-carboxylate; 3-Pyridinecarboxylic acid, 6-((3,4-dihydro-4,4-dimethyl-2H-1-benzothiopyran-6-yl)ethynyl)-, ethyl ester; 3-Pyridinecarboxylic acid, 6-((3,4-dihydro-4,4-dimethyl-2H-1-benzothiopyran-6-yl)ethynyl)-,ethyl ester
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Dermatologic Agents
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
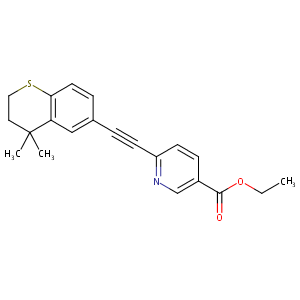 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
