Details of the Drug Combinations
General Information of This Drug (ID: DMCL2OK)
| Drug Name | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Abstenisil; Abstinil; Abstinyl; Alcophobin; Antabus; Antabuse; Antadix; Antaenyl; Antaethan; Antaethyl; Antaetil; Antalcol; Antetan; Antethyl; Antetil; Anteyl; Anthethyl; Antiaethan; Anticol; Antietanol; Antiethanol; Antietil; Antikol; Antivitium; Aversan; Averzan; Bonibal; Contralin; Contrapot; Cronetal; Dicupral; Disetil; Disulfan; Disulfirame; Disulfiramo; Disulfiramum; Disulfirm; Disulfram; Disulfuram; Disulphuram; Ephorran; Espenal; Esperal; Etabus; Ethyldithiourame; Ethyldithiurame; Exhoran; Exhorran; Gababentin; Hoca; Krotenal; Nocbin; Nocceler; Noxal; Refusal; Stopaethyl; Stopethyl; Stopety; Stopetyl; TATD; TETD; THIOCID; TTD; TTS; Tenurid; Tenutex; Tetidis; Tetradin; Tetradine; Tetraethylthiuram; Tetraetil; Teturam; Teturamin; Thireranide; Thiuranide; Tillram; Tiuram; Accel TET; Akrochem TETD; Ancazide ET; Antab use; Disulfirame [DCIT]; Ekagom DTET; Ekagom TEDS; Ekagom TETDS; Ekaland TETD; Esperal [France]; Eta bus; Ethyl Thiram; Ethyl Thiudad; Ethyl Thiurad; Ethyl Tuads Rodform; Ethyl Tuex; Ethyl tuads; Etyl Tuex; Nocceler TET; Perkacit TETD; Perkait TETD; Robac TET; Sanceler TET; Soxinol TET; TTS x; Tet raethylthiuram; Thiuram E; Dupon 4472; T 1132; Accel TET-R; Alk-aubs; Antabus (TN); Antabuse (TN); Anti-ethyl; Antivitium (Spain); ENT 27,340; Nocceler TET-G; Noxal (VAN); Ro-sulfiram; Sanceler TET-G; Tuads, ethyl; Usaf B-33; Ro-Sulfram-500 (USA)
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Alcohol Deterrents
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
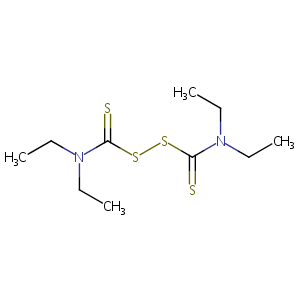 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
6 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
