Details of the Drug Combinations
General Information of This Drug (ID: DMF8Y74)
| Drug Name | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Atara; Atarax; Ataraxoid; Atarazoid; Atarox; Atazina; Aterax; Deinait; Durrax; Hidroxizina; Hychotine; Hydroksyzyny; Hydroxine; Hydroxizine; Hydroxizinum; Hydroxycine; Hydroxyzin; Hydroxyzinum; Hydroxyzyne; Idrossizina; Neurozina; Nevrolaks; Orgatrax; Pamazone; Parenteral; Paxistil; Placidol; Plaxidol; Tranquizine; Traquizine; Vistaril; Atarax base; Hydroksyzyny [Polish]; Hydroxyzine Hcl; Hydroxyzine base; Hydroxyzine hydrochloride; Idrossizina [DCIT]; NP 212; UCB 4492; UCB 492; Atarax (TN); Hidroxizina [INN-Spanish]; Hy-Pam 25; Hydroxyzine (INN); Hydroxyzine [INN:BAN]; Hydroxyzinum [INN-Latin]; Marex (TN); Neo-Calma; Tran-Q; U.CB 4492; Vesparaz-Wirkstoff; Vistaril (TN); U.C.B 4492; N-(4-Chlorobenzhydryl)-N'-(hydroxyethoxyethyl)piperazine; N-(4-Chlorobenzhydryl)-N'-(hydroxyethyloxyethyl)piperazine; Ethanol, 2-(2-(4-((4-chlorophenyl)phenylmethyl)-1-piperazinyl)ethoxy)-(9CI); 1-(p-Chloro-.alpha.-phenylbenzyl)-4-[2-[(2-hydroxyethoxy)ethyl]piperazine; 1-(p-Chloro-alpha-phenylbenzyl)-4-(2-(2-hydroxyethoxy)ethyl)piperazine; 1-(p-Chloro-alpha-phenylbenzyl)-4-(2-hydroxyethoxyethyl)piperazine; 1-(p-Chlorobenzhydryl)-4-(2-(2-hydroxyethoxy)ethyl)diethylenediamine; 1-(p-Chlorobenzhydryl)-4-(2-(2-hydroxyethoxy)ethyl)piperazine; 1-(p-Chlorobenzhydryl)-4-[2-(2-hydroxyethoxy)ethyl]diethylenediamine; 1-(p-Chlorobenzhydryl)-4-[2-(2-hydroxyethoxy)ethyl]piperazine; 1-(p-Chlorodiphenylmethyl)-4-(2-(2-hydroxyethoxy)ethyl)piperazine; 1-(p-Chlorodiphenylmethyl)-4-[2-(2-hydroxyethoxy)ethyl]piperazine; 2-(2-(4-((4-Chlorophenyl)phenylmethyl)-1-piperazinyl)ethoxy)ethanol; 2-(2-(4-(p-Chloro-alpha-phenylbenzyl)-1-piperazinyl)ethoxy)ethanol; 2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-1-yl}ethoxy)ethanol; 2-[(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-1-yl}ethyl)oxy]ethanol; 2-[2-[4-(p-Chloro-.alpha.-phenylbenzyl)-1-piperazinyl]ethoxy]ethanol; 2-[2-[4-[(4-chlorophenyl)-phenylmethyl]piperazin-1-yl]ethoxy]ethanol
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Hypnotics and Sedatives
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
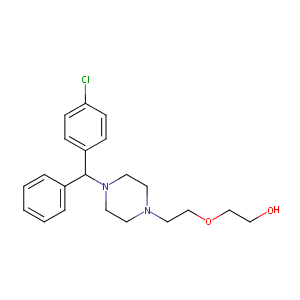 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
3 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||
References
