Details of the Drug Combinations
General Information of This Drug (ID: DMHN027)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Eltroxin; Euthyrox; Forthyron; Laevothyroxinum; Laevoxin; Levolet; Levothroid; Levothyrox; Levothyroxin; Levoxyl; Oroxine; Synthroid; THX; Tetraiodothyronine; Thyratabs; Thyrax; Thyreoideum; Thyroxevan; Thyroxin; Thyroxinal; Levothyroxine sodium; Synthroid sodium; Thyroxine [BAN]; Thyroxine iodine; LT00440967; T4 levothyroxine; DL-Thyroxin; Eltroxin (TN); Euthyrox (TN); Eutirox (TN); Forthyron (TN); L-Thryoxin; L-Thyroxin; L-thyroxine; Laevothyroxinum (acid); Levaxin (TN); Levo-T; Levothyroxine (BAN); Levothyroxinum (acid); Levoxyl (TN); Synthroid (TN); T-3850; T4 (Hormone); Thyrax (TN); Thyrox (TN); Thyroxine (VAN); Thyroxine (l); L-thyroxine (TN); Levothyroxine Sodium (L-thyroxine); Levothroid (*Sodiumsalt*); Synthroid (*Sodium salt*); Thyroxine, L-(8CI); L-3,5,3',5'-Tetraiodothyronine; O-(4-Hydroxy-3,5-diiodophenyl)-3,5-diiodotyrosine; Beta-((3,5-Diiodo-4-hydroxyphenoxy)-3,5-diiodophenyl)alanine; O-(4-Hydroxy-3,5-diidophenyl)-3,5-diiodo-L-tyrosine; O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-L-tyrosine; (-)-Thyroxine; (125I)T4; (2S)-2-amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]propanoic acid; 2-amino-3-[4-(4-hydroxy-3,5-diiodo-phenoxy)-3,5-diiodo-phenyl]-propanoic acid; 3,3',5,5"-Tetraiodo-L-thyronine; 3,3',5,5''-Tetraiodo-L-thyronine; 3,3',5,5'-Tetraiodo-L-thyronine; 3,5,3',5'-Tetraiodo-L-thyronine; 3,5,3',5'-Tetraiodothyronine; 3,5,3'5'-Tetraiodo-L-thyronine; 3-(4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl)alanine; 3-[4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]-L-alanine; 4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodo-L-phenylalanine
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antithyroid Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
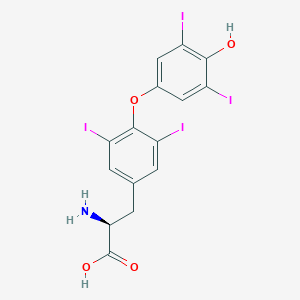 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
4 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||
References
