Details of the Drug Combinations
General Information of This Drug (ID: DMJIBAW)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Bidien; Budenofalk; Budeson; Budesonido; Budesonidum; Budiair; Cortivent; Entocort; Horacort; Inflammide; Micronyl; Miflonide; Preferid; Pulmaxan; Respules; Rhinocort; Spirocort; UDB; Budecort Inhaler; Budesonide Easyhaler; Budesonide MMX; Entocort EC; GionaEasyhaler; Pulmaxan turbohaler; Pulmicort Flexhaler; Pulmicort Nebuamp; Pulmicort Respules; Pulmicort Topinasal; Pulmicort turbuhaler; Rhinocort Aqua; Rhinocort Turbuhaler; Rhinocort alpha; Unit dosebudesonide; B 7777; S 1320; Budesonido [INN-Spanish]; Budesonidum [INN-Latin]; Entocort (TN); Entocort EC (TN); MAP-0010; Noex (TN); Pulmicort (TN); Rhinocort (TN); S-1320; Budesonide (JAN/USAN/INN); Budesonide [USAN:INN:BAN:JAN]; Rhinocort, Pulmicort , Entocort, Symbicort, Noex. Entocort EC, Budesonide; Pregna-1,4-diene-3,20-dione, 16,17-butylidenebis(oxy)-11,21-dihydroxy-, (11beta,16alpha(R))-,and 16alpha,17-((S)-Butylidenebis(oxy))-11beta,21-dihydroxypregna-1,4-diene-3,20-dione; (11-beta,16-alpha)-16,17-(Butylidenebis(oxy))-11,21-dihydroxypregna-1,4-diene-3,20-dione; (11beta,16alpha)-16,17-(Butylidenebis(oxy))-11,21-dihydroxypregna-1,4-diene-3,20-dione; (4aR,4bS,5S,6aS,6bS,8R,9aR,10aS,10bS)-5-hydroxy-6b-(hydroxyacetyl)-4a,6a-dimethyl-8-propyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one; (4aR,4bS,5S,6aS,6bS,9aR,10aS,10bS)-5-hydroxy-6b-(hydroxyacetyl)-4a,6a-dimethyl-8-propyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one; (R,S)-11b,16a,17,21,tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with buty raldehyde; (RS)-(11beta,16alpha)-16,17-[Butylidenebis(oxy)]-11,21-dihydroxypregna-1,4-diene-3,20-dione; (RS)-11beta,16alpha,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with butyraldehyde; (S)-16alpha,17-(Butylidenedioxy)-11beta,21-dihydroxypregna-1,4-diene-3,20-dione; 11beta,21-dihydroxy-16alpha,17alpha-(butane-1,1-diyldioxy)pregna-1,4-diene-3,20-dione; 16,17-Butylidenebis(oxy)-11,21-dihydroxypregna-1,4-diene-3,20-dione; 16,17-Butylidenebis(oxy)-11-,21-dihydroxypregna-1,4-diene-3,20-dione; 16-alpha,17-alpha-Butylidenedioxy-11-beta,21-dihydroxy-1,4-pregnadiene-3,20-dione; 16alpha(R),17-(Butylidenebis(oxy))-11beta,21-dihydroxypregna-1,4-diene-3,20-dione; 16alpha-,17alpha-butylidenedioxypregna-1,4-diene-11beta-,21-diol-3,20-dione
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiinflammatory Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
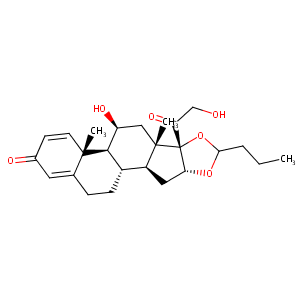 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
14 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
1 Approved Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
