Details of the Drug Combinations
General Information of This Drug (ID: DMSIMD8)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Anaptivan; Biociclin; Biofuroksym; Bioxima; CXM; Cefofix; Cefumax; Cefurex; Cefuril; Cefuroxim; Cefuroximesodium; Cefuroximine; Cefuroximo; Cefuroximum; Cephuroxime; Cetroxil; Colifossim; Curocef; Curoxim; Curoxima; Curoxime; Froxal; Furoxil; Kesint; Ketocef; Lifurox; Medoxim; Sharox; Spectrazolr; Ultroxim; Zinacef;CEFUROXIME AND DEXTROSE IN DUPLEX CONTAINER; CEFUROXIME SODIUM; Cefuroxim AJ; Cefuroxim Fresenius; Cefuroxim Genericsn; Cefuroxim Hexal; Cefuroxim Lilly; Cefuroxim MN; Cefuroxim Norcox; Cefuroxim curasan; Cefuroxima Fabra; Cefuroxima Richet; Cefuroxime for Injection and Dextrose for Injection in Duplex Container; Cefuroxime na; Cefuroxime sodium salt; KEFUROX IN PLASTIC CONTAINER; Sodium cefuroxime; ZINACEF IN PLASTIC CONTAINER; Zinacef Danmark; Ceftin (TN); Cefuroxim Norcox [inj.]; Cefuroxime (TN); Cefuroximo [INN-Spanish]; Cefuroximum [INN-Latin]; Cetroxil [inj.]; Froxal [inj.]; KS-1040; Sharox [inj.]; Zinacef (TN); Zinnat (TN); Zinnat [inj.]; Cefuroxime (USAN/INN); Cefuroxime [USAN:INN:BAN]; Cefuroxime sodium (JP15/USP); Cefuroxime sodium [USAN:BAN:JAN]; Sodium (6R-(6alpha,7beta(Z)))-3-(((aminocarbonyl)oxy)methyl)-7-(2-furyl(methoxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylate; Sodium (6R,7R)-7-(2-(2-furyl)glyoxylamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylate, 7(sup 2)-(Z)-(O-methyloxime), carbamate (ester); (6R,7R)-3-(carbamoyloxymethyl)-7-[[(2Z)-2-(furan-2-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-[(carbamoyloxy)methyl]-7-[(2Z)-2-(furan-2-yl)-2-(methoxyimino)acetamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-3-[(carbamoyloxy)methyl]-7-{[(2Z)-2-furan-2-yl-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-(2-(2-Furyl)glyoxylamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid 7(sup 2)-(Z)-(O-methyloxime) carbamate (ester); 3-[(carbamoyloxy)methyl]-7beta-[(2Z)-2-(furan-2-yl)-2-(methoxyimino)acetamido]-3,4-didehydrocepham-4-carboxylic acid
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
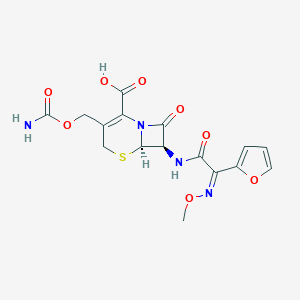 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
4 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||
References
