Details of the Drug Combinations
General Information of This Drug (ID: DMSZQAK)
| Drug Name | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | Amphetamine (tamper and abuse-resistant, Bio-MD/MPAR, ADHD); Amphetamine (tamper and abuse-resistant, Bio-MD/MPAR, ADHD), PharmacoFore; PF-08 (Bio-MD/MPAR/prodrug/oral, ADHD), PharmacoFore | ||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
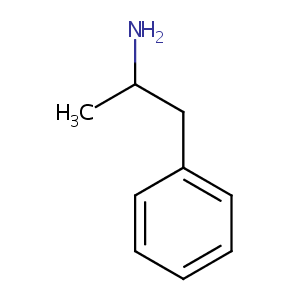 |
||||||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
