Details of the Drug Combinations
General Information of This Drug (ID: DMUOK4C)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acerbon; Acercomp; Alapril; Carace; Cipral; Cipril; Coric; Doneka; Hipril (TN); Inhibril; Inopril; LPR; Linopril; Linvas; Lipril; Lisinal; Lisinopril (INN); Lisinopril (anhydrous); Lisinopril anhydrous; Lisinoprilum; Lisinoprilum [Latin]; Lisipril; Lisoril; Lispril; Longes; Loril; Lysinopril; MK 521; MK 522; MK-521; Noperten; Novatec; Presiten; Prinil; Prinivil; Prinivil (TN); Sinopril; Sinopryl; Tensopril; Tensopril (TN); Tensyn; Tersif; Vivatec; Zestril; Zestril (TN)
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
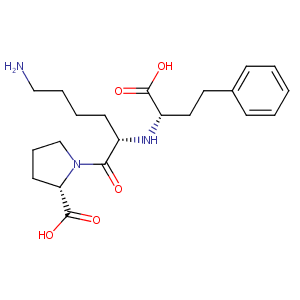 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
13 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
