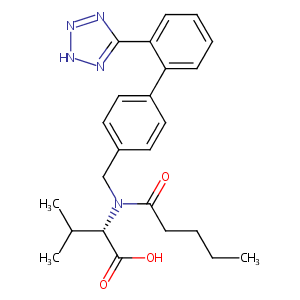
| Synonyms |
valsartan; 137862-53-4; Diovan; Tareg; Provas; L-Valsartan; CGP 48933; Exforge; CGP-48933; (S)-2-(N-((2'-(1H-Tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)pentanamido)-3-methylbutanoic acid; UNII-80M03YXJ7I; N-(p-(o-1H-Tetrazol-5-ylphenyl)benzyl)-N-valeryl-L-valine; CHEMBL1069; 80M03YXJ7I; CHEBI:9927; C24H29N5O3; (2S)-3-methyl-2-[pentanoyl-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]amino]butanoic acid; N-(1-oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-L-valine; 137863-60-6; AK-58790; Kalpress; Miten; Nisis; Diovan; Vals; Valsarran; Walsartan; Aventis brand of valsartan; CEPA brand of valsartan; Esteve brand of valsartan; Lacer brand of valsartan; Novartis brand of valsartan; Sanol brand of valsartan; Schwarz brand of valsartan; Diovan (TN); Diovan, Valsartan; Valsartan [USAN:INN]; Valtan (TN); Valzaar (TN); Valsartan (JAN/USAN/INN); N-valeryl-N-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)valine; N-pentanoyl-N-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-L-valine; N-pentanoyl-N-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl}-L-valine; L-Valine, N-(1-oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-(9CI); (S)-N-valeryl-N-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]-methyl}-valine; (s)-2-(n-((2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methyl)pentanamido)-3-methylbutanoic acid; [3H]valsartan
|