Details of the Drug
General Information of Drug (ID: DM1CI9R)
| Drug Name |
Bempedoic acid
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ETC-1002; bempedoic acid; 738606-46-7; 8-Hydroxy-2,2,14,14-tetramethylpentadecanedioic acid; ESP-55016; UNII-1EJ6Z6Q368; ETC 1002; Bempedoic acid; ESP-55016; 1EJ6Z6Q368; Bempedoate; Bempedoic acid [USAN:INN]; ETC1002; ESP 55016; Bempedoic acid (USAN/INN); SCHEMBL185768; GTPL8321; CHEMBL3545313; HYHMLYSLQUKXKP-UHFFFAOYSA-N; EX-A1243; ZINC3948738; BCP16083; s7953; AKOS027439916; CS-3952; DB11936; AK499358; AC-29040; AS-49804; HY-12357; Bempedoic Acid(ETC-1002;ESP-55016); D10691; Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl-
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
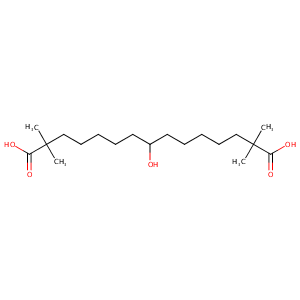 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 344.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.8 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 14 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Bempedoic acid (Comorbidity)
|
|||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | ||||
|---|---|---|---|---|---|
| 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 3 | FDA Approved Products: Nexletol (bempedoic acid) oral tablets | ||||
| 4 | Bempedoic Acid (ETC-1002): A Current Review. Cardiol Clin. 2018 May;36(2):257-264. doi: 10.1016/j.ccl.2017.12.007. Epub 2018 Feb 21. | ||||
| 5 | Walker CJ, Cowan DA, James VH, Lau JC, Kicman AT: Doping in sport: 3. Metabolic conversion of oral norethisterone to urinary 19-norandrosterone. Steroids. 2009 Mar;74(3):341-9. doi: 10.1016/j.steroids.2008.11.008. Epub 2008 Nov 19. | ||||
| 6 | Product Information. Nexletol (bempedoic acid). Esperion Therapeutics, Ann Arbor, MI. | ||||
