Details of the Drug
General Information of Drug (ID: DM1ECNJ)
| Drug Name |
GS-7340
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | Tenofovir alafenamide; Vemlidy | ||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
HIV-1Herpes simplex virusHepatitis B virus
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
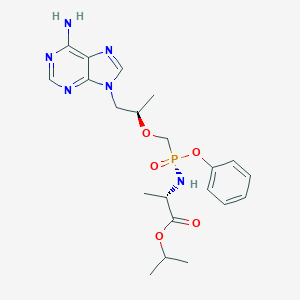 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 476.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 12 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2020 | ||||
|---|---|---|---|---|---|
| 2 | ClinicalTrials.gov (NCT01565850) Safety and Efficacy of Darunavir/Cobicistat/Emtricitabine/GS-7340 Single Tablet Regimen Versus Cobicistat-boosted Darunavir Plus Emtricitabine/Tenofovir Disoproxil Fumarate Fixed Dose Combination in HIV-1 Infected, Antiretroviral Treatment Naive Adults. U.S. National Institutes of Health. | ||||
