Details of the Drug
General Information of Drug (ID: DM2BO9W)
| Drug Name |
BCP-13498
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Propacetamol; Pro-Dafalgan; Propacetamol (INN); Propacetamol [INN:BAN]; Propacetamol [INN]; Propacetamolum; Propacetamolum [Latin]; Tox21_113811; ZINC55161176; (4-acetamidophenyl) 2-(diethylamino)acetate; 5CHW4JMR82; 66532-85-2; AC1L2ALY; AC1Q6199; AKOS015890722; BCP13498; CHEBI:135089; CHEMBL1851805; DB09288; DSSTox_CID_31589; DSSTox_GSID_57800; DSSTox_RID_97473; DTXSID3057800; EINECS 266-390-1; Glycine, N,N-diethyl-, 4-(acetylamino)phenyl ester; N,N-Diethylglycine, ester with 4'-hydroxyacetanilide; SCHEMBL26155; UNII-5CHW4JMR82
|
|||||
| Affected Organisms |
Humans and other mammals
|
|||||
| ATC Code | ||||||
| Structure |
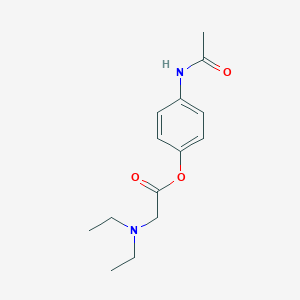 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 264.32 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 1.1 | |||||
| Rotatable Bond Count (rotbonds) | 7 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
