Details of the Drug
General Information of Drug (ID: DM65AGJ)
| Drug Name |
Oxymorphone
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | Oxymorphone (abuse-resistant, pain); Oxymorphone (abuse-resistant, pain), Pain Therapeutics/ King Pharmaceuticals; Oxymorphone (abuse-resistant, pain), Pain Therapeutics/Pfizer | ||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Neurology Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
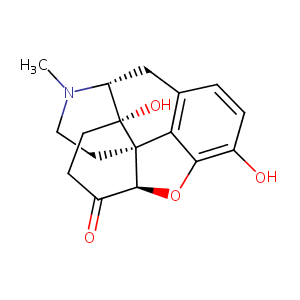 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 301.34 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.8 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Oxymorphone
Coadministration of a Drug Treating the Disease Different from Oxymorphone (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | Oxymorphone FDA Label | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7094). | ||||
| 7 | Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Br J Clin Pharmacol. 2010 Jul;70(1):78-87. | ||||
| 8 | Product Information. Buprenex (buprenorphine). Reckitt and Colman Pharmaceutical, Richmond, VA. | ||||
| 9 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 10 | US Food and Drug Administration "FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines requires its strongest warning.". | ||||
| 11 | Vizcaychipi MP, Walker S, Palazzo M "Serotonin syndrome triggered by tramadol." Br J Anaesth 99 (2007): 919. [PMID: 18006535] | ||||
| 12 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 13 | Product Information. Apadaz (acetaminophen-benzhydrocodone). KemPharm, Inc, Coralville, IA. | ||||
| 14 | Product Information. Suprep Bowel Prep Kit (magnesium/potassium/sodium sulfates). Braintree Laboratories, Braintree, MA. | ||||
| 15 | Product Information. Zulresso (brexanolone). Sage Therapeutics, Inc., Cambridge, MA. | ||||
| 16 | Product Information. Reyvow (lasmiditan). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 17 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 18 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 19 | Cerner Multum, Inc. "Canadian Product Information.". | ||||
