Details of the Drug
General Information of Drug (ID: DMA5Q4T)
| Drug Name |
2-methoxy-1,4-naphthoquinone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
2-Methoxy-1,4-naphthoquinone; 2348-82-5; 2-Methoxynaphthoquinone; 2-methoxynaphthalene-1,4-dione; 2-Methoxy-p-naphthoquinone; 1,4-Naphthalenedione, 2-methoxy-; 1,4-NAPHTHOQUINONE, 2-METHOXY-; UNII-39020BUT1D; 2-Methoxy-1,4-naphthalenedione; NSC 31530; AI3-17893; CHEMBL106562; 2-Methoxy-[1,4]naphthoquinone; CHEBI:69522; OBGBGHKYJAOXRR-UHFFFAOYSA-N; 39020BUT1D; 2-methoxy-1,4-dihydronaphthalene-1,4-dione; ACMC-20ans6; AC1L28UU; MLS002472995; 2-methoxy-1,4-napthoquinone; SCHEMBL571061; AC1Q483I; DTXSID1062338; CTK4F1565
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
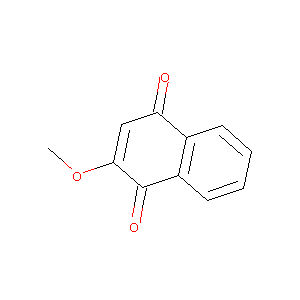 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 188.18 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
