Details of the Drug
General Information of Drug (ID: DMDZE8N)
| Drug Name |
CAPROCTAMINE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CAPROCTAMINE; CHEMBL11805; SCHEMBL18398791; BDBM50067482; N,N''''-(octane-1,8-diyl)bis(6-((2-methoxybenzyl)(methyl)amino)-N-methylhexanamide); 6-[(2-Methoxy-benzyl)-methyl-amino]-hexanoic acid [8-({6-[(2-methoxy-benzyl)-methyl-amino]-hexanoyl}-methyl-amino)-octyl]-methyl-amide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
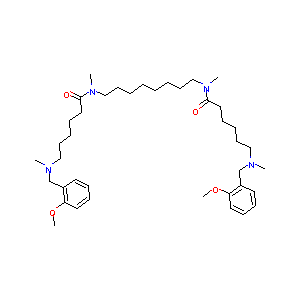 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 667 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 27 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
