Details of the Drug
General Information of Drug (ID: DMFE3CT)
| Drug Name |
Alniditan
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-B57Z82EOGE; B57Z82EOGE; 152317-89-0; [3H]alniditan; Alniditan [INN:BAN]; N-[[(2R)-chroman-2-yl]methyl]-N'-(1,4,5,6-tetrahydropyrimidin-2-yl)propane-1,3-diamine; AC1Q4UFC; AC1L24EQ; GTPL117; GTPL120; SCHEMBL717887; CHEMBL88240; ZINC1536693; PDSP2_001383; PDSP1_001399; BDBM50403503; CS-6739; HY-101698; 2-((3-(((R)-2-Chromanylmethyl)amino)propyl)amino)-1,4,5,6-tetrahydropyrimidine; Pasmigren; R-091274
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
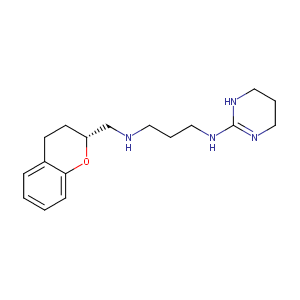 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 302.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
