Details of the Drug
General Information of Drug (ID: DMHI7CQ)
| Drug Name |
JDQ443
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
JDQ-443; Opnurasib; JDQ443; 2653994-08-0; NVP-JDQ443; NVP-JDQ-443; JDQ 443; (S)-JDQ-443; 1-[6-[4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methylindazol-5-yl)pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl]prop-2-en-1-one; opnurasib [INN]; Q3W0H3V1LQ; CHEMBL5077861; JDQ 443 [WHO-DD]; SCHEMBL23533580; GTPL11715; GLXC-25533; EX-A5693; BDBM50579985; NSC846146; AKOS040757949; AT36708; compound 5 [PMID: 35404998]; HY-139612A; NSC-846146; example 1a [WO2021120890A1]; MS-29737; CS-0226220; CS-0311034; -PROPEN-1-ONE, 1-(6-((4R)-4-(5-CHLORO-6-METHYL-1H-INDAZOL-4-YL)-5-METHYL-3-(1-METHYL-1H-INDAZOL-5-YL)-1H-PYRAZOL-1-YL)-2-AZASPIRO(3.3)HEPT-2-YL)-; 1-(6-((4S)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl)-2-azaspiro[3.3]heptan-2-yl)prop-2-en-1-one; 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-inda zol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]- 2-Propen-1-one; 1-[6-[(4R)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-2-propen-1-one; 1-{6-[(4M)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5- methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2- azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 1-{6-[(4M)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 2-Propen-1-one, 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-; 2653994-10-4
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
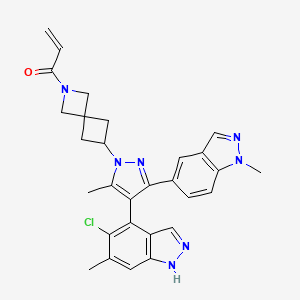 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
