Details of the Drug
General Information of Drug (ID: DMJFP6G)
| Drug Name |
Metergolin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Metergoline; metergoline; Liserdol; 17692-51-2; Methergoline; Metergolina; Metergolinum; Metergolina [DCIT]; Metergolinum [Latin]; Metergolina [Spanish]; Substance 355/255; Metergoline [INN:BAN]; Metergolinum [INN-Latin]; 1-Methyl-N-carbobenzyloxydihydro-D-lysergamin; FI-6337; MLS000069437; UNII-1501393LY5; C25H29N3O2; AHR 3009; 1-Methyl-8-beta-carbobenzyloxyaminomethyl-10-alpha-ergoline; EINECS 241-686-3; 1,6-Dimethyl-8-beta-carbobenzyloxaminomethyl-10-alpha-ergoline; FI 6337; Liserdol (TN)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
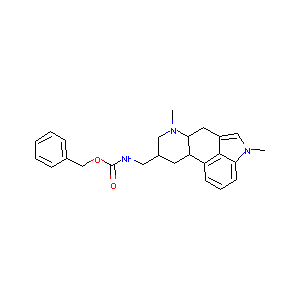 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 403.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
