Details of the Drug
General Information of Drug (ID: DML6CN4)
| Drug Name |
Acemetacin
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Acemetacina; Acemetacina [INN-Spanish]; Acemetacine; Acemetacine [INN-French]; Acemetacinum; Acemetacinum [INN-Latin]; Acemix; Aximeixin; Emflex; Rantudil; Rheumibis; TVX 3322; indometacin carboxymethyl ester; indometacin glycolic ester; indomethacin carboxymethyl ester; indomethacin glycolic ester; 1H-Indole-3-acetic acid, 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-, carboxymethyl ester; 53164-05-9; BRN 0501672; Bay f 4975; C21H18ClNO6; CHEBI:31162; CHEMBL189171; EINECS 258-403-4; K 708; K-708; UNII-5V141XK28X; acemetacin
|
|||||
| Affected Organisms |
Humans and other mammals
|
|||||
| ATC Code | ||||||
| Structure |
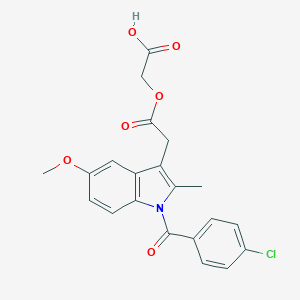 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 415.8 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | |||||
| Rotatable Bond Count (rotbonds) | 7 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
