Details of the Drug
General Information of Drug (ID: DMN0LP8)
| Drug Name |
Polymyxin B Sulfate
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aerosporin; Polimixina B; Polymixin B sulfate; Polymxin B sulfate; Polymyxin b; Polymyxine B; Polymyxinum B; Terramycin Ophthalmic; Polimixina B [INN-Spanish]; Polymyxin B Sulfate [USAN:JAN]; Polymyxin B, sulfate; Polymyxine B [INN-French]; Polymyxinum B [INN-Latin]; Aerosporin, PMB, Poly-RX,Polymyxin B sulphate; 4-amino-N-[1-[[4-amino-1-oxo-1-[[6,9,18-tris(2-aminoethyl)-15-benzyl-3-(2-hydroxyethyl)-12-(2-methylpropyl)-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptazacyclotricos-21-yl]amino]butan-2-yl]amino]-4-hydroxy-1-oxobutan-2-yl]-2-formamidobutanamide
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
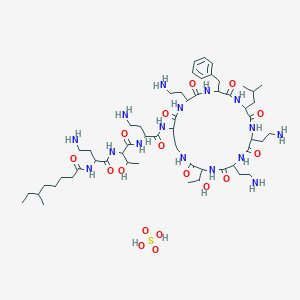 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 |
Molecular Weight | 1301.6 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count | 29 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count | 20 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 22 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
