Details of the Drug
General Information of Drug (ID: DMOJA5G)
| Drug Name |
Nuplazid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pimavanserin tartrate; UNII-NA83F1SJSR; 706782-28-7; ACP 103; ACP-103; 706782-28-7 (tartrate); NA83F1SJSR; Pimavanserin tartrate [USAN]; Bis(1-(4-Fluorobenzyl)-1-(1-methylpiperidin-4-yl)-3-(4-(2-methylpropoxy)benzyl)urea) (2R,3R)-2,3-dihydroxybutanedioate; Pimavanserin tartrate (USAN); 1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)-1-(1-methylpiperidin-4-yl)urea, ((2R,3R)-2,3-dihydroxysuccinate) (2:1); Nuplazide (TN); pimavanserin hemitartrate; DTXSID50220958; CHEBI:133014; HMS3886L06; HY-14557A; Pimavanserin Dihydroxysuccinate(2:1); AKOS027327334; CCG-270608; CS-7954; 1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)-1-(1-methylpiperidin-4-yl)urea (2R,3R)-2,3-dihydroxysuccinate; AC-29901; AS-56699; N-(4-Fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1); Urea, N-((4-fluorophenyl)methyl)-N-(1-methyl-4-piperidinyl)-N'-((4-(2-methylpropoxy)phenyl)methyl)-, (2R,3R)-2,3-dihydroxybutanedioate (2:1); D08969; Q27284759; bis(4-{[(4-fluorophenyl)methyl]({[4-(2-methylpropoxy)phenyl]methyl}carbamoyl)amino}-1-methylpiperidin-1-ium) (2R,3R)-2,3-dihydroxybutanedioate; bis{N-[(4-fluorophenyl)methyl]-N-(1-methylpiperidin-4-yl)-N'-{[4-(2-methylpropoxy)phenyl]methyl}urea} (2R,3R)-2,3-dihydroxybutanedioate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
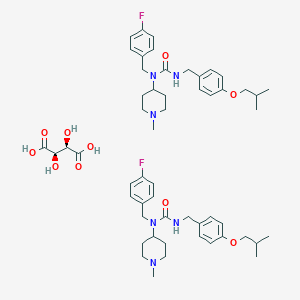 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 |
Molecular Weight | 1005.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 19 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 6 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 14 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Alzheimer disease | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A20 | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
References
