Details of the Drug
General Information of Drug (ID: DMT6AM2)
| Drug Name |
Ozanimod
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1306760-87-1; Ozanimod (RPC1063); UNII-Z80293URPV; Z80293URPV; (S)-5-(3-(1-((2-hydroxyethyl)amino)-2,3-dihydro-1H-inden-4-yl)-1,2,4-oxadiazol-5-yl)-2-isopropoxybenzonitrile; Ozanimod [USAN:INN]; Ozanimod (USAN/INN); SCHEMBL2195490; GTPL8709; CHEMBL3707247; 5-[3-[(1S)-1-(2-hydroxyethylamino)-2,3-dihydro-1H-inden-4-yl]-1,2,4-oxadiazol-5-yl]-2-propan-2-yloxybenzonitrile; EX-A1316; s7952; ZINC116109867; AKOS026674086; DB12612; CS-5070; HY-12288; AC-29883; Benzonitrile, 5-(3-((1
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Structure |
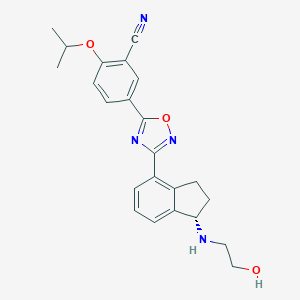 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 404.5 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Ozanimod (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | ||||
|---|---|---|---|---|---|
| 2 | ClinicalTrials.gov (NCT02294058) Phase 3 Study of RPC1063 in Relapsing MS. U.S. National Institutes of Health. | ||||
| 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 4 | FDA Approved Products: Zeposia (Ozanimod) oral capsules | ||||
| 5 | Results From the First-in-Human Study With Ozanimod, a Novel, Selective Sphingosine-1-Phosphate Receptor Modulator. J Clin Pharmacol. 2017 Aug;57(8):988-996. doi: 10.1002/jcph.887. Epub 2017 Apr 11. | ||||
| 6 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 7 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 8 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 9 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 10 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
