Details of the Drug
General Information of Drug (ID: DMUTL7O)
| Drug Name |
Cloxacillin
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Chloroxacillin; Clossacillina; Cloxacilina; Cloxacilline; Cloxacillinum; MCIPC; Orbenin; Syntarpen; Tegopen; Clossacillina [DCIT]; Cloxacillin sodium; Methocillin S; BRL 1621; Cloxacilina [INN-Spanish]; Cloxacillin (INN); Cloxacillin [INN:BAN]; Cloxacilline [INN-French]; Cloxacillinum [INN-Latin]; Cloxapen (TN); Cloxapen (sodium monohydrate); Methylchlorphenylisoxazoryl-penicillin; Novo-Cloxin; Nu-Cloxi; Orbenin (TN); Tegopen (sodium monohydrate); BRL-1621 (sodium monohydrate); P-25 (sodium monohydrate); [3-(O-CHLOROPHENYL)-5-METHYL-4-ISOXAZOLYL]PENICILLIN; (2S,5R,6R)-6-({[3-(2-chlorophenyl)-5-methylisoxazol-4-yl]carbonyl}amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-6-[[3-(2-chlorophenyl)-5-methyl-1,2-oxazole-4-carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (3-(o-Chlorophenyl)-5-methyl-4-isoxazolyl)penicillin; 6-(((3-(2-Chlorophenyl)-5-methyl-4-isoxazolyl)carbonyl)amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid; 6-(3-(o-Chlorophenyl)-5-methyl-4-isoxazolecarboxamido)penicillanic acid
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
||||||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
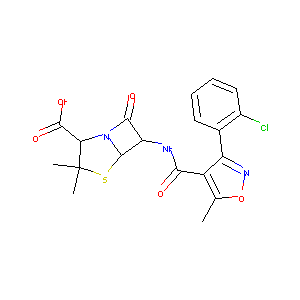 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 435.9 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.4 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 061454. | ||||
|---|---|---|---|---|---|
| 2 | Cloxacillin FDA Label | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Hartman NR, Mao JJ, Zhou H, Boyne MT 2nd, Wasserman AM, Taylor K, Racoosin JA, Patel V, Colatsky T: More methemoglobin is produced by benzocaine treatment than lidocaine treatment in human in vitro systems. Regul Toxicol Pharmacol. 2014 Oct;70(1):182-8. doi: 10.1016/j.yrtph.2014.07.002. Epub 2014 Jul 8. | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Development of a receptor-based microplate assay for the detection of beta-lactam antibiotics in different food matrices. Anal Chim Acta. 2007 Mar 14;586(1-2):296-303. | ||||
| 7 | Potential cholestatic activity of various therapeutic agents assessed by bile canalicular membrane vesicles isolated from rats and humans. Drug Metab Pharmacokinet. 2003;18(1):16-22. | ||||
| 8 | Pro-inflammatory cytokines enhance dilatation of bile canaliculi caused by cholestatic antibiotics. Toxicol In Vitro. 2019 Aug;58:51-59. doi: 10.1016/j.tiv.2019.03.015. Epub 2019 Mar 12. | ||||
