Details of the Drug
General Information of Drug (ID: DMWFU3T)
| Drug Name |
1-(3-Fluoro-pyridin-2-yl)-4-methyl-piperazine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
85386-90-9; 1-(3-fluoropyridin-2-yl)-4-methylpiperazine; CHEMBL280514; 1-Methyl-4-(3-fluoro-2-pyridinyl)piperazine; 1-(3-Fluoro-pyridin-2-yl)-4-methyl-piperazine; Piperazine, 1-(3-Fluoro-2-pyridinyl)-4-methyl- (9ci); SCHEMBL10964078; KZNJAAWCEFRAPW-UHFFFAOYSA-N; ZINC36237989; BDBM50026633; AKOS025286419; AM87258
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
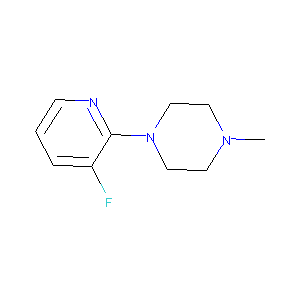 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 195.24 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
