| Synonyms |
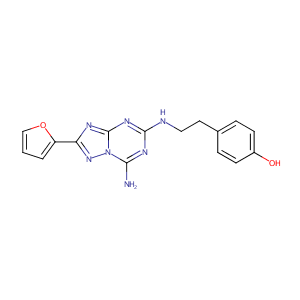
ZM241385; 139180-30-6; ZM 241385; ZM-241385; CHEMBL113142; 4-(2-(7-Amino-2-(2-furyl)(1,2,4)triazolo(2,3-a)(1,3,5)triazin-5-ylamino)ethyl)phenol; 4-(2-[7-Amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol; 4-(2-(7-Amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-5-ylamino)ethyl)phenol; 4-{2-[(7-Amino-2-Furan-2-Yl[1,2,4]triazolo[1,5-A][1,3,5]triazin-5-Yl)amino]ethyl}phenol; 4-[2-[[7-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-5-yl]amino]ethyl]phenol
|
| Chemical Identifiers |
- Formula
- C16H15N7O2
- IUPAC Name
4-[2-[[7-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-a][1,3,5]triazin-5-yl]amino]ethyl]phenol - Canonical SMILES
-
C1=COC(=C1)C2=NN3C(=NC(=NC3=N2)NCCC4=CC=C(C=C4)O)N
- InChI
-
InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22)
- InChIKey
-
PWTBZOIUWZOPFT-UHFFFAOYSA-N
|