Details of the Drug
General Information of Drug (ID: DMZ43OM)
| Drug Name |
PMID26560530-Compound-25
|
|||||
|---|---|---|---|---|---|---|
| Affected Organisms |
Humans and other mammals
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Structure |
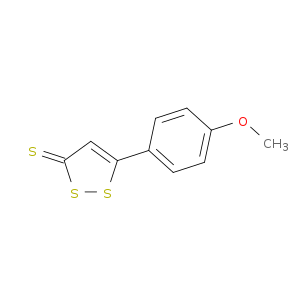 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 240.4 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | |||||
| Rotatable Bond Count (rotbonds) | 2 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
References
