Details of the Drug Combination
General Information of Drug Combination (ID: DC3CYMQ)
| Drug Combination Name |
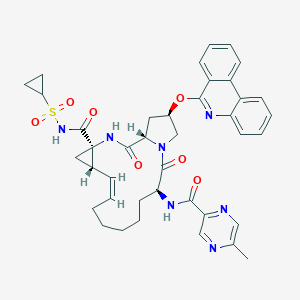
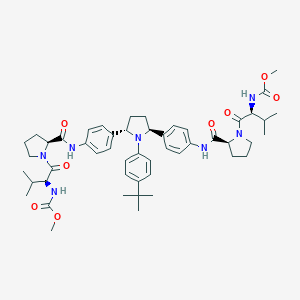
Paritaprevir Ombitasvir
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication |
|
|||||||||||||||||
| Component Drugs | Paritaprevir | Ombitasvir | ||||||||||||||||
| Small molecular drug | Small molecular drug | |||||||||||||||||
|
|
|||||||||||||||||
| 2D MOL | 2D MOL | |||||||||||||||||
| 3D MOL | 3D MOL | |||||||||||||||||
Molecular Interaction Atlas of This Drug Combination
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication(s) of Paritaprevir |
|
|||||||||||||||||||||||||
|
Paritaprevir Interacts with 1 DTP Molecule(s)
|
||||||||||||||||||||||||||
| Indication(s) of Ombitasvir |
|
|||||||||||||||||||||||||
|
Ombitasvir Interacts with 1 DTP Molecule(s)
|
||||||||||||||||||||||||||
Test Results of This Drug Combination in Other Disease Systems
|
|||||||||||||||||||||||||
References
