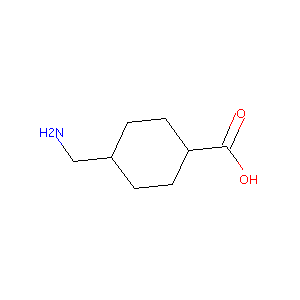
| 1 |
ClinicalTrials.gov (NCT02458729) The Blood Saving Effect of Tranexamic Acid in Total Knee Arthroplasty With Rivaroxaban as Thromboprophylaxis
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6573).
|
| 3 |
Tranexamic acid FDA Label
|
| 4 |
Tranexamic Acid (TXA) and Corona Virus 2019 (COVID19) in Inpatients (TCInpatient)
|
| 5 |
Evaluation of aprotinin and tranexamic acid in different in vitro and in vivo models of fibrinolysis, coagulation and thrombus formation. J Thromb Haemost. 2007 Oct;5(10):2113-8.
|
|
|
|
|
|
|