Details of the Drug
General Information of Drug (ID: DMFI8A7)
| Drug Name |
Tranexamic Acid
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
AMCA; AMCHA; AMH; Amikapron; Amstat; Anvitoff; Carxamin; Cyclocapron; Cyklokapron; Emorhalt; Exacyl; Frenolyse; Hexapromin; Hexatron; Mastop; Rikavarin; Spiramin; Tamcha; Tranex; Tranexamsaeure; Tranexan; Transamin; Transamlon; Trasamlon; Ugurol; Acide tranexamique; Acido tranexamico; Acidum tranexamicum; Tranexmic acid; Tranhexamic acid; Trans AMCHA; CL 65336; DV 79; DV79; KABI 2161; LT00159441; ALBB-006013; Acide tranexamique [INN-French]; Acido tranexamico [INN-Spanish]; Acidum tranexamicum [INN-Latin]; CL-65336; Cis-AMCHA; Cyklokapron (TN); DV-79; RP 18,429; Rikavarin (TN); Rikavarin-S; T-AMCHA; Tranexamic acid cis-form; Trans-Amcha; Trans-Tranexamic acid; Cis-4-(Aminomethyl)cyclohexanecarboxylic acid; Tranexamic acid (JP15/USAN/INN); Tranexamic acid [USAN:INN:BAN:JAN]; Trans-4-(Aminomethyl)cyclohexanecarboxylic acid; Trans-4-(Aminomethyl)cyclohexanecarboxylic acid ester; Trans-p-(Aminomethyl)cyclohexanecarboxylic; Trans-p-(Aminomethyl)cyclohexanecarboxylic acid; Cis-4-Aminomethylcyclohexane-1-carboxylic acid; Trans-1-Aminomethylcyclohexane-4-carboxylic acid; Trans-4-(Aminomethyl)cyclohexane-carboxylic acid; Trans-4-Aminomethylcyclohexane-1-carboxylic acid; Trans-1-(Aminomethyl)cyclohexane-4-carboxylic acid; Trans-4-(Aminomethyl)-1-cyclohexanecarboxylic acid; Trans-4-(Aminomethyl)cyclohexane-1-carboxylic acid; 4-(Aminomethyl)-Cyclohexanecarboxylic Acid; 4-(Aminomethyl)cyclohexanecarboxylic acid; 4-(aminomethyl)cyclohexane-1-carboxylic acid
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antifibrinolytic Agents
|
||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||
| Structure |
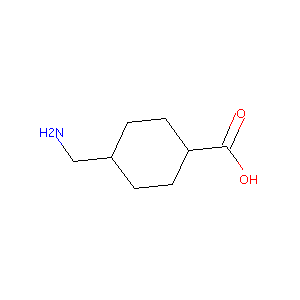 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 157.21 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Excessive bleeding | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | GA30.02 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Tranexamic Acid (Comorbidity)
|
|||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6573). | ||||
|---|---|---|---|---|---|
| 2 | Tranexamic acid FDA Label | ||||
| 3 | Tranexamic Acid (TXA) and Corona Virus 2019 (COVID19) in Inpatients (TCInpatient) | ||||
| 4 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Evaluation of aprotinin and tranexamic acid in different in vitro and in vivo models of fibrinolysis, coagulation and thrombus formation. J Thromb Haemost. 2007 Oct;5(10):2113-8. | ||||
| 7 | Cerner Multum, Inc. "Australian Product Information.". | ||||
