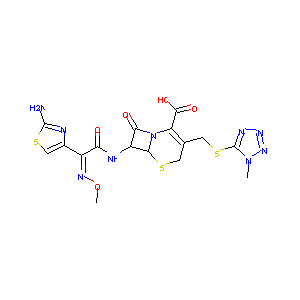
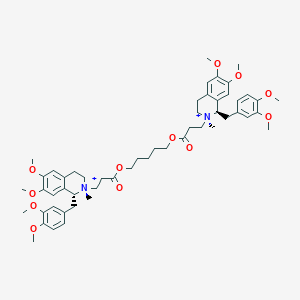
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
In vitro evaluation of E1040, a new cephalosporin with potent antipseudomonal activity. Antimicrob Agents Chemother. 1988 May;32(5):693-701.
|
| 5 |
Bradycardia produced by pyridostigmine and physostigmine. Can J Anaesth. 1997 Dec;44(12):1286-92.
|
| 6 |
Pharmacological characteristics of the inhibition of nondepolarizing neuromuscular blocking agents at human adult muscle nicotinic acetylcholine receptor. Anesthesiology. 2009 Jun;110(6):1244-52.
|
|
|
|
|
|
|