Details of the Drug
General Information of Drug (ID: DMUZPJ5)
| Drug Name |
Cisatracurium
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | Cisatracurium Besylate; Cisatracurium Besylate Preservative Free; Nimbex; Nimbex Preservative Free | ||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
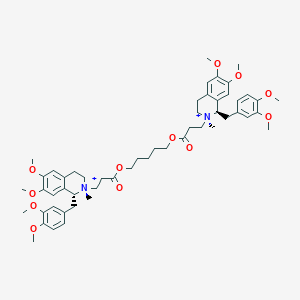 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 929.1 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 7.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 26 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 12 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Cisatracurium
Coadministration of a Drug Treating the Disease Different from Cisatracurium (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | Bradycardia produced by pyridostigmine and physostigmine. Can J Anaesth. 1997 Dec;44(12):1286-92. | ||||
| 6 | Pharmacological characteristics of the inhibition of nondepolarizing neuromuscular blocking agents at human adult muscle nicotinic acetylcholine receptor. Anesthesiology. 2009 Jun;110(6):1244-52. | ||||
| 7 | Pandit SK, Ferres CJ, Gibson FM, Mirakhur RK "Time course of action of combinations of vecuronium and pancuronium." Anaesthesia 41 (1986): 151-4. [PMID: 2869710] | ||||
| 8 | Alahdal O, Bevan DR "Clindamycin-induced neuromuscular blockade." Can J Anaesth 42 (1995): 614-7. [PMID: 7553999] | ||||
| 9 | Fuchs-Buder T, Wilder-Smith OH, Borgeat A, Tassonyi E "Interaction of magnesium sulphate with vecuronium-induced neuromuscular block." Br J Anaesth 74 (1995): 405-9. [PMID: 7734259] | ||||
| 10 | Burkett L, Bikhazi GB, Thomas KC Jr, Rosenthal DA, Wirta MG, Foldes FF "Mutual potentiation of the neuromuscular effects of antibiotics and relaxants." Anesth Analg 58 (1979): 107-15. [PMID: 571233] | ||||
| 11 | Multum Information Services, Inc. Expert Review Panel. | ||||
| 12 | Product Information. Camptosar (irinotecan). Pharmacia and Upjohn, Kalamazoo, MI. | ||||
| 13 | Product Information. Alfenta (alfentanil). Janssen Pharmaceutica, Titusville, NJ. | ||||
| 14 | Liberman BA, Norman P, Hardy BG "Pancuronium-phenytoin interaction: a case of decreased duration of neuromuscular blockade." Int J Clin Pharmacol Ther Toxicol 26 (1988): 371-4. [PMID: 3220609] | ||||
| 15 | Chapple DJ, Clark JS, Hughes R "Interaction between atracurium and drugs used in anaesthesia." Br J Anaesth 55 Suppl 1 (1983): s17-22. [PMID: 6688011] | ||||
| 16 | Cushard WG, Kohanim M, Lantis LR "Blastomycosis of bone." J Bone Joint Surg Am 51A (1969): 704-12. [PMID: 5819086] | ||||
| 17 | Harrah MD, Way WL, Katzung BG "The interaction of d-tubocurarine with antiarrhythmic drugs." Anesthesiology 33 (1970): 406-10. [PMID: 5512329] | ||||
| 18 | Katende RS, Dimich I "Resistance to nondepolarizing muscle relaxants in a patient treated with ranitidine." Mt Sinai J Med 54 (1987): 330-1. [PMID: 2955219] | ||||
| 19 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 20 | Huang KC, Heise A, Shrader AK, Tsueda K "Vancomycin enhances the neuromuscular blockade of vecuronium." Anesth Analg 71 (1990): 194-6. [PMID: 1973882] | ||||
