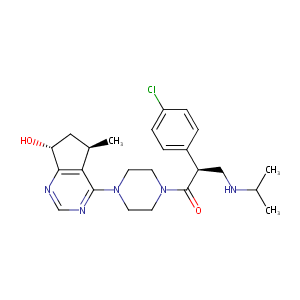
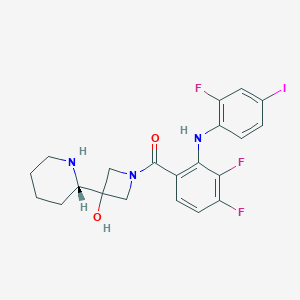
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 3 |
Clinical pipeline report, company report or official report of Roche.
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800031868)
|
| 6 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 7 |
ClinicalTrials.gov (NCT01106599) A Study Evaluating the Safety, Tolerability, and Pharmacokinetics of GDC-0623 in Patients With Locally Advanced or Metastatic Solid Tumors. U.S. National Institutes of Health.
|
| 8 |
PI3K/AKT inhibitors aggravate death receptor-mediated hepatocyte apoptosis and liver injury. Toxicol Appl Pharmacol. 2019 Oct 15;381:114729. doi: 10.1016/j.taap.2019.114729. Epub 2019 Aug 22.
|
|
|
|
|
|
|